Abstract
Constitutively active JAK2V617F and thrombopoietin receptor (TpoR) W515L/K mutants are major determinants of human myeloproliferative neoplasms (MPNs). We show that a TpoRW515 mutation (W515A), which we detected in 2 myelofibrosis patients, and the Δ5TpoR active mutant, where the juxtamembrane R/KW515QFP motif is deleted, induce a myeloproliferative phenotype in mouse bone marrow reconstitution experiments. This phenotype required cytosolic Y112 of the TpoR. Phosphotyrosine immunoprofiling detected phosphorylated cytosolic TpoR Y78 and Y112 in cells expressing TpoRW515A. Mutation of cytosolic Y112 to phenylalanine prevented establishment of the in vivo phenotype and decreased constitutive active signaling by Δ5TpoR and TpoRW515A, especially via the mitogen-activated protein (MAP)–kinase pathway, without decreasing Janus kinase 2 (JAK2) activation. In contrast, mutation of cytosolic Y78 to phenylalanine enhanced the myeloproliferative syndrome induced by the TpoRW515 mutants, by enhancing receptor-induced JAK2 activation. We propose that TpoR cytosolic phosphorylated Y112 and flanking sequences could become targets for pharmacologic inhibition in MPNs.
Introduction
The thrombopoietin receptor (c-Mpl or TpoR) and its ligand Tpo are the main regulators of megakaryocyte differentiation and platelet production.1-3 TpoR also plays an important role in hematopoietic stem cell (HSC) renewal, quiescence, and expansion.4-10
Signaling by TpoR depends on the cytoplasmic Janus kinase 2 (JAK2),11-15 which also regulates receptor traffic and stability on the cell surface.14 Tpo binding to the extracellular domain is thought to induce, like erythropoietin binding to the erythropoietin receptor (EpoR),16,17 dimerization of receptor cytosolic domains into a configuration that promotes cross-phosphorylation and activation of JAK2. In turn, activated JAK2 phosphorylates tyrosine residues in the receptor cytoplasmic domain and provides docking sites for downstream signaling molecules such as signal transducer and activator of transcription 3 (STAT3), Src homologous and collagen (Shc), growth factor receptor–bound protein 2, phosphatidylinositol-3′-kinase (PI-3-kinase), and possibly STAT5. Three TpoR cytosolic tyrosine residues have been shown to play important roles in signaling. Y78 (numbering corresponds to cytosolic residues only), functions as a negative regulatory site,18,19 whereas the distal tyrosine residues, Y112 and Y117, function as positive regulatory sites.18 Y112 is essential for mitogen-activated protein (MAP)–kinase activation through recruitment of the Shc adaptor and for recruitment or activation of STAT5, STAT3, Shc-associated p145 (SHIP), Src homology 2 domain-containing protein tyrosine phosphatase-2, and Gab2.18-22
Analysis of juxtamembrane sequences in EpoR, and in the TpoR, led to the notion that these residues play crucial roles in promoting physiologic activation, but also in maintaining unliganded receptors inactive.23-25 EpoR as well as the growth hormone receptor have been described as preformed dimers that, upon ligand binding, change conformation via rotation and scissors-like movements.26-28 Whereas the cytosolic domains of cytokine receptors are flexible,29 the juxtamembrane domains, and especially the cytosolic juxtamembrane sequences, appear to be rigid and to contain important residues for transmitting the receptor active conformation to intracellular JAK2.17,30
Consistent with an important regulatory role of receptor juxtamembrane sequence, we have recently shown that the deletion of 5 amino acid residues, K/RWQFP, at the outset of the cytosolic juxtamembrane region results in a mutant TpoR, Δ5TpoR, that activates downstream signaling pathways in the absence of its ligand Tpo.25 Mutation of W515 (W508 in mice, W515 in humans) to alanine suffices to activate the receptor. We therefore suggested that the K/RWQFP motif and especially residue W515 could be target residues for activating mutations in humans. Indeed, a fraction (5%–7%) of patients who suffer from primary myelofibrosis (PMF) and essential thrombocytosis harbor point mutations in the K/RWQFP motif, resulting in amino acid substitutions of W515 by either leucine or lysine.31,32 Furthermore, the transforming activity of TpoRW515K was shown to require cell surface localization and dimerization of the receptor.33 Mouse bone marrow adoptive transfer experiments have shown that the W515L mutant induces a rapid and severe myeloproliferative disorder associated with myelofibrosis, which resembles myeloproliferative neoplasm (MPN) with myelofibrosis in patients.31 Here we describe 2 primary myelofibrosis patients who harbor a TpoRW515A mutant and show that both TpoRW515A and the Δ5TpoR mutant induce a severe and rapid myeloproliferative disorder in mouse bone marrow transplantation assays that was mediated via Y112 of the TpoR cytosolic domain. We show by phosphotyrosine immunoprofiling and mass spectrometry that 2 cytosolic TpoR residues, Y78 and Y112, are constitutively phosphorylated in TpoRW515A-expressing cell lines. Importantly, substitution of Y112 by phenylalanine abolishes pathologic signaling and the induction in vivo of a myeloproliferative phenotype, whereas mutation of Y78 to phenylalanine induces a more severe phenotype. Y112F inhibits downstream signaling, but not JAK2 activation, whereas Y78F enhances JAK2 activation by TpoR. Because Y112 is not absolutely required for the physiologic function of TpoR in vivo,34 but is crucial for the pathologic phenotype induced by TpoRW515 mutants, our results suggest that pathologic signaling via Y112 might be a novel target for inhibition by small molecules in MPN patients with TpoRW515 mutations.
Methods
Genomic sequencing of patient cells
The study was approved by the Local Research Ethics Committee of all participating institutions with informed consent obtained in accordance with the Declaration of Helsinki. Clinical diagnosis of PMF was established according to the Italian criteria for PMF. Patient information collection, blood sample management, erythrocyte removal, and granulocyte purification were performed, as described.35 Total blood DNA and granulocyte DNA were purified using QIAGEN kits (QIAGEN). Direct sequencing of genomic DNA was performed by first subjecting samples to polymerase chain reaction,35 and then resulting polymerase chain reaction products were purified on a QIAGEN column and subjected to sequencing on an ABI PRISM 3100 Genetic Analyser (Applied Biosystems).
cDNA constructs
Generation of cell lines
To obtain Ba/F3 cell lines stably expressing the TpoRwt or the various TpoR mutants, retroviral supernatants were generated as described.25 Populations of cells expressing green fluorescent protein (GFP) at levels above 25% were isolated by fluorescence-activated cell sorting sorting. For bone marrow reconstitution experiments, pMEGIX-IRES-GFP containing constructs were packaged using vesicular stomatitis virus G envelope protein in 293-EBNA packaging cells.38
Assay for Tpo-independent and Tpo-dependent growth
Cells were washed with RPMI medium, then placed either in RPMI medium containing 10% fetal bovine serum either without cytokines or with the indicated concentrations of recombinant murine Tpo (R&D Systems). Cell numbers were counted using a Coulter Counter after the indicated intervals.
Cell proliferation assay
The cell proliferation assay was performed using a CellTiter 96 Non-Radioactive Cell Proliferation Assay (Promega). The assay was carried out according to the manufacturer's protocol. Briefly, cells were plated with 5000 cells/well in RPMI containing 10% fetal bovine serum. Absorbance was measured at 570 nm, which is directly proportional to the number of living cells in culture.
Surface expression of HA-tagged wild-type TpoR and Δ5TpoR
Surface expression of HA-TpoR proteins was measured in Ba/F3 cells and in primary bone marrow reconstituted mouse blood cells by flow cytometry using monoclonal anti-HA antibody (10 μg/mL; Covance) and R-phycoerythrin–conjugated donkey F(ab′)2 anti–mouse immunoglobulin G secondary antibody (12.5 μg/mL) as described.25
Immunoblots
Ba/F3 cells expressing the TpoRwt or various TpoR mutants were starved from cytokines and serum for 5 hours in RPMI containing 1 mg/mL bovine serum albumin. Cells were stimulated with 50 ng/mL Tpo for 15 minutes at 37°C or left untreated. Cells were lysed in 2× Laemmli sample buffer and passed through a 28-gauge needle 10 times to shear DNA. Samples were then separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (10% gel) and blotted onto a cellulose membrane (Hybond-C super membrane; Amersham Life Science).
An antibody directed to phosphorylated TpoR Y112 (Y616 or Y626, full mouse and human TpoR numbering, respectively) was raised against a peptide where Y112 was phosphorylated. The sequence is identical between the mouse and human receptors and recognizes both human and mouse TpoR phosphorylated at Y112 in Western blot assays.
Western blotting antibodies were directed against HA (Roche), JAK2 (Upstate Biotechnology Inc), β-actin (Sigma), phospho-JAK2 (Tyr1007/1008), phospho-Akt (Ser473), phospho-STAT1 (Tyr701), phospho-STAT3 (Tyr705), phospho-STAT5 (Tyr694), phospho–extracellular signal-regulated kinase 1/2 (Erk1/2; Tyr202/204), and phospho-p38 MAP-kinase (Thr180/Tyr182; Cell Signaling Technology).
Bone marrow reconstitution assays and histopathology
Bone marrow cells were isolated from mice 3 days after 5-fluorouracil injection, as described.37,38 Retrovirally transduced cells (106) were injected into 6- to 8-week-old C57Bl6 lethally irradiated mice and peripheral blood was examined for GFP expression 3 weeks after transplantation. Peripheral blood cell counts were performed using a Melet Schloessing MS9-3 cell counter. For histopathology analysis, femurs and spleens were fixed in formaldehyde, decalcified, and paraffin embedded. Sections (4.5 μm) were stained with hematoxylin-eosin, trichrome, and Giemsa. Reticulin fibers were revealed by silver staining according to the Gordon Sweet method. May-Grünwald-Giemsa staining was performed on blood smears and on bone marrow and spleen imprints.
Phosphotyrosine scanning and mass spectrometry
Phosphopeptides were prepared using PhosphoScan Kit (Cell Signaling Technology). Briefly, approximately 2 × 108 cells were lysed in urea buffer; trypsin-digested lysates were purified by Sep-pak C18 column. Then, lyophilized peptides were redissolved and immunoaffinity purified with pY-100 antibody. pTyr-containing peptides were concentrated on reverse-phase microtips. Liquid chromatography tandem mass spectrometry analysis was performed as previously described.39
Statistical analysis
Results are presented as means (± SD) as indicated, and data were analyzed with the 2-tailed Student t test. In figures, P values less than .05 are indicated by an asterisk.
Results
Identification of TpoRW515A in patients with myelofibrosis
Our previous structural work indicated that the R/KWQFP motif forms an amphipathic α-helix, where W515 impairs close apposition of cytosolic domains in a productive dimeric conformation permissive for activation of JAK2.25 Because a variety of mutations at W515 (W515A, W515L, W515K, W515S)25,31,32,40 lead to constitutive TpoR activation, we hypothesized that MPD patients harbor mutations at W515 other than the previously reported TpoRW515L and W515K mutations.
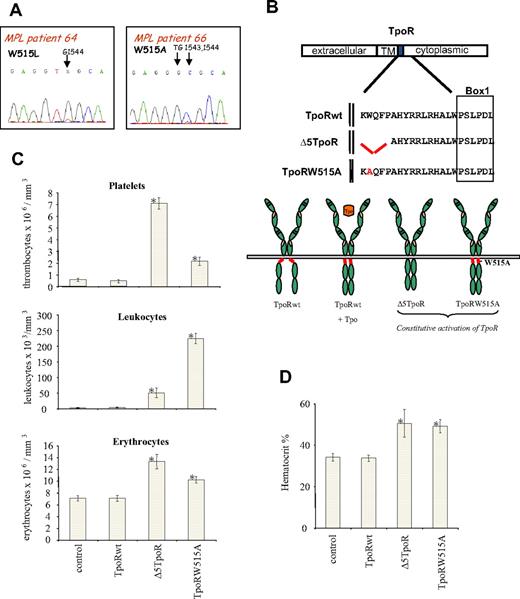
Using genomic sequencing of granulocyte DNA from PMF patients, we indeed identified 2 patients who were heterozygous for W515A; the sequence chromatogram for one of the patients depicting the TGG (Trp)→GCG (Ala) mutation is shown in Figure 1A. In the same figure, the sequence of a PMF patient who harbors the described TGG (Trp)→TTG (Leu) mutation is shown. Both patients were negative for the JAK2V617F mutation.
Myeloproliferative disorder induced in vivo by Δ5TpoR and TpoRW515A mutants. (A) Sequencing of genomic DNA isolated from peripheral blood granulocytes of primary myelofibrosis patients. A W515L (TGG→TTG) mutation was identified in TpoR from patient 64. A W515A (TGG→GCG) mutation was detected in TpoR from patient 66. (B) Shown is the location of the amphipathic K(R)WQFP motif at the junction between the TpoR transmembrane (TM) and cytosolic domains. (C) Peripheral blood counts, taken 45 days after reconstitution with bone marrow cells expressing the indicated TpoR variants, demonstrated that Δ5TpoR and TpoRW515A induce a rapid myeloproliferative disease. (D) Hematocrit values (%) were determined 45 days for mice reconstituted with bone marrow cells expressing the indicated TpoR constructs.
Myeloproliferative disorder induced in vivo by Δ5TpoR and TpoRW515A mutants. (A) Sequencing of genomic DNA isolated from peripheral blood granulocytes of primary myelofibrosis patients. A W515L (TGG→TTG) mutation was identified in TpoR from patient 64. A W515A (TGG→GCG) mutation was detected in TpoR from patient 66. (B) Shown is the location of the amphipathic K(R)WQFP motif at the junction between the TpoR transmembrane (TM) and cytosolic domains. (C) Peripheral blood counts, taken 45 days after reconstitution with bone marrow cells expressing the indicated TpoR variants, demonstrated that Δ5TpoR and TpoRW515A induce a rapid myeloproliferative disease. (D) Hematocrit values (%) were determined 45 days for mice reconstituted with bone marrow cells expressing the indicated TpoR constructs.
In vivo expression of the Δ5TpoR or the TpoRW515A
We generated vesicular stomatitis virus G pseudotyped retrovirus using a bicistronic mouse stem cell virus (pMEGIX)–based vector where the gene of interest was cloned upstream of the internal ribosomal entry site (IRES) and the expression marker green fluorescent protein (GFP). The level of expression of the gene cloned upstream of the IRES correlates with the level of GFP downstream of the IRES and expression levels can be monitored by fluorescence-activated cell sorting analysis.36,38
We performed bone marrow transplantations with hematopoietic cells retrovirally transduced with the empty vector as control, the wild-type TpoR (TpoRwt), the Δ5TpoR, or TpoRW515A (Figure 1B). Peripheral nucleated blood cells from all examined mice were GFP positive (> 95%) starting 3 weeks after reconstitution.
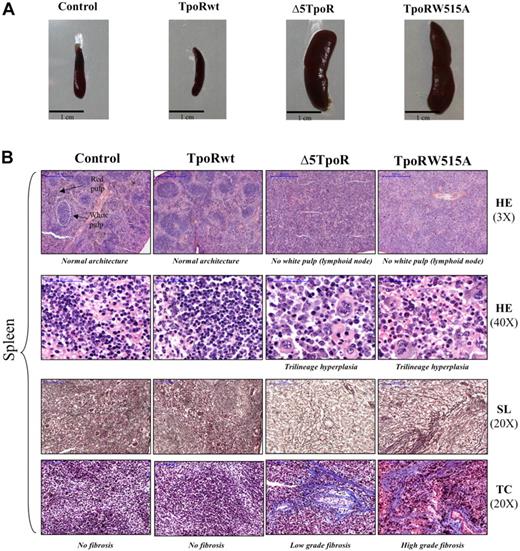
Peripheral blood counts recorded 45 days after reconstitution showed that mice expressing the Δ5TpoR had elevated levels of white blood cells and platelets (Figure 1C). In addition, cell counts for the erythroid lineage were slightly elevated (Figure 1C), along with a significant increase of the hematocrit compared with the mice expressing either the empty vector or TpoRwt (Figure 1D). Mice expressing the TpoRW515A mutant show similar features as Δ5TpoR-expressing mice. In contrast, mice reconstituted with TpoRwt-expressing cells had similar blood cell counts as control mice and did not develop any disease (Figure 1C). Examination of bone marrow (supplemental Figure 1B, available on the Blood website; see the Supplemental Materials link at the top of the online article) and spleen (Figure 2B) histology of Δ5TpoR and TpoRW515A mice showed predominant megakaryocyte proliferation with morphologic changes depicted in supplemental Figure 1B, namely anisocytosis (ranging from small hypolobulated to larger hyperlobulated megakaryocytes), abnormal nuclear-cytoplasmic ratio, abnormal chromatin clumping, emperipolesis, and paratrabecular localization. This was associated with myeloid hyperplasia in the bone marrow, and with increased erythropoiesis and megakaryopoiesis in the spleen (Figure 2B). Extramedullary hematopoiesis was present with erythroid and granulocytic precursor cells in the liver (supplemental Figure 1A). Furthermore, mice expressing Δ5TpoR and TpoRW515A exhibited marked splenomegaly (Figure 2A) with a complete disappearance of the white pulp (lymphoid tissue; Figure 2B). Silver (SL) or trichrome (TC) staining clearly showed the presence of a weak fibrosis (spleen, liver, and bone marrow) in Δ5TpoR mice (Figure 2A and supplemental Figure 1A-B). More severe fibrosis was observed in TpoRW515A mice, suggesting that TpoRW515A has a stronger activity than Δ5TpoR (Figure 2B).
Histology of mice reconstituted with bone marrow–expressing TpoR variants. Bone marrow cells were infected with empty vector pMEGIX, or pMEGIX viruses coding for wild-type TpoR (TpoRwt), Δ5TpoR, or TpoRW515A mutants. (A) Splenomegaly was detected in mice expressing the active TpoR mutants compared with control empty vector or TpoRwt mice. All mice present more than 95% GFP-positive cells at 3 weeks after reconstitution. (B) Histology of spleen sections of mice reconstituted with the indicated constructs. Spleen sections are stained with hematoxylin-eosin (HE; 3× or 40× magnification), silver stain (SL; 20× magnification), or trichrome (TB; 20× magnification). Spleen sections were performed 45 days after transplantation. Microscopic section of the spleen shows expansion of the red pulp (HE, 3×). The splenic enlargement is due mainly to extramedullary hematopoiesis (HE, 40×) as well as fibrosis (SL, 20× and TC, 20×) that occur in the splenic red pulp sinusoids. Megakaryocytes are prominent, but erythropoiesis and granulopoiesis are present as well in the splenic sinuses. Both Δ5TpoR and TpoRW515A induce some degree of early fibrosis with reticulin (see silver staining: SL, 20×) and collagen deposition stained in blue by the Masson trichrome (TC, 20×). Images were obtained using a Mirax Digital Slide System.
Histology of mice reconstituted with bone marrow–expressing TpoR variants. Bone marrow cells were infected with empty vector pMEGIX, or pMEGIX viruses coding for wild-type TpoR (TpoRwt), Δ5TpoR, or TpoRW515A mutants. (A) Splenomegaly was detected in mice expressing the active TpoR mutants compared with control empty vector or TpoRwt mice. All mice present more than 95% GFP-positive cells at 3 weeks after reconstitution. (B) Histology of spleen sections of mice reconstituted with the indicated constructs. Spleen sections are stained with hematoxylin-eosin (HE; 3× or 40× magnification), silver stain (SL; 20× magnification), or trichrome (TB; 20× magnification). Spleen sections were performed 45 days after transplantation. Microscopic section of the spleen shows expansion of the red pulp (HE, 3×). The splenic enlargement is due mainly to extramedullary hematopoiesis (HE, 40×) as well as fibrosis (SL, 20× and TC, 20×) that occur in the splenic red pulp sinusoids. Megakaryocytes are prominent, but erythropoiesis and granulopoiesis are present as well in the splenic sinuses. Both Δ5TpoR and TpoRW515A induce some degree of early fibrosis with reticulin (see silver staining: SL, 20×) and collagen deposition stained in blue by the Masson trichrome (TC, 20×). Images were obtained using a Mirax Digital Slide System.
Immunoprofiling of tyrosine-phosphorylated proteins from cells expressing TpoRW515A
The PhosphoScan approach identifies the phosphotyrosine component of the phosphoproteome and reveals large numbers of tyrosine phosphorylation sites. Peptides containing phosphotyrosine are isolated directly from protease-digested cellular protein extracts with a phosphotyrosine-specific antibody and are identified by tandem mass spectrometry.41 This strategy allows the identification of activated protein kinases and their phosphorylated substrates without prior knowledge of the signaling networks that are activated. This approach has successfully been used for identification of oncogenic signaling and has led to identification of an active mutant of JAK3 in megakaryocytic leukemic cell lines39 and to a common phosphotyrosine signature for the Bcr-Abl tyrosine kinase signature.42
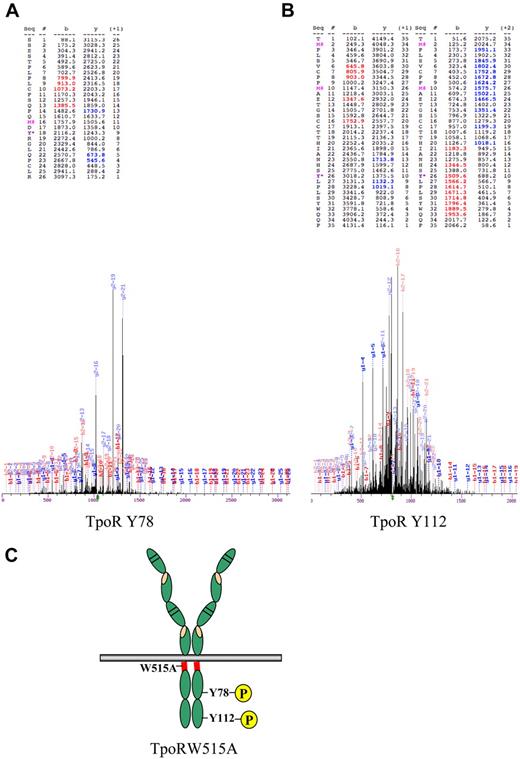
We therefore examined the profile of phosphorylated peptides between parental Ba/F3 cells, and Ba/F3 cells that express TpoRW515A after cell sorting (sorted) or after selection for autonomous growth (selected). As shown in Figure 3, 2 peptides that belonged to the TpoR sequence were phosphorylated in TpoRW515A cells selected for autonomous growth. One contained phosphorylated Y78 (Figure 3A) and the other contained phosphorylated Y112 (Figure 3B). Interestingly, for the peptide that encompasses phosphorylated Y112, the downstream Y117 residue (also present in the trypsin-digested peptide) was detected in nonphosphorylated form. Aside from TpoR tyrosine-phosphorylated sites, phosphorylated peptides belonging to STAT5, STAT3, Erk1/2, and p38 were detected (extensive list in supplemental Table 1). For all these proteins, the phosphorylated tyrosine residues were the sites known to be required for activation of signaling (Y694 for STAT5, Y701 for STAT3), consistent with detection by Western blotting with phospho-specific antibodies of the same sites in Figure 6. Taken together, these data show that Y112 and Y78 are phosphorylated in cells rendered autonomous by TpoRW515A.
Evidence that Y78 and Y112 of TpoR are phosphorylated in TpoRW515A cells selected for autonomous growth. Phosphopeptides were prepared using PhosphoScan Kit (Cell Signaling Technology) after lysis of Ba/F3 TpoRW515A cells in urea buffer. Trypsin-digested lysates were immunoaffinity purified with pY-100 antibody, concentrated on reverse-phase microtips, and analyzed by mass spectrometry. (A) Mass spectrum representing phosphorylation of Y78 of TpoR (Y582, full murine TpoR numbering). (B) Mass spectrum representing phosphorylation of Y112 of TpoR (Y616, full murine TpoR numbering). Oxidized methionine is represented by M# and tyrosine phosphorylation, by Y*. Phosphorylated residues are denoted as y. (C) Schematic representation of TpoR with the location of the W515A mutation in the TpoR cytosolic juxtamembrane domain (red region) and the Y78 and Y112 in the cytosolic domain of the receptor.
Evidence that Y78 and Y112 of TpoR are phosphorylated in TpoRW515A cells selected for autonomous growth. Phosphopeptides were prepared using PhosphoScan Kit (Cell Signaling Technology) after lysis of Ba/F3 TpoRW515A cells in urea buffer. Trypsin-digested lysates were immunoaffinity purified with pY-100 antibody, concentrated on reverse-phase microtips, and analyzed by mass spectrometry. (A) Mass spectrum representing phosphorylation of Y78 of TpoR (Y582, full murine TpoR numbering). (B) Mass spectrum representing phosphorylation of Y112 of TpoR (Y616, full murine TpoR numbering). Oxidized methionine is represented by M# and tyrosine phosphorylation, by Y*. Phosphorylated residues are denoted as y. (C) Schematic representation of TpoR with the location of the W515A mutation in the TpoR cytosolic juxtamembrane domain (red region) and the Y78 and Y112 in the cytosolic domain of the receptor.
Role of the TpoR cytoplasmic tyrosine residues in the induction of the myeloproliferative phenotype by Δ5TpoR and TpoRW515A
We then investigated whether Y78 and Y112 are involved in the transforming ability of the Δ5TpoR or TpoRW515A. Mutants were engineered, where Y78 (full receptor numbering is Y582 for murine TpoR and Y592 for human TpoR) or Y112 (full receptor numbering is Y616 for mouse TpoR, and Y626 for human TpoR; for schema see Figure 3C) was individually replaced by phenylalanine.
Δ5TpoR mutants
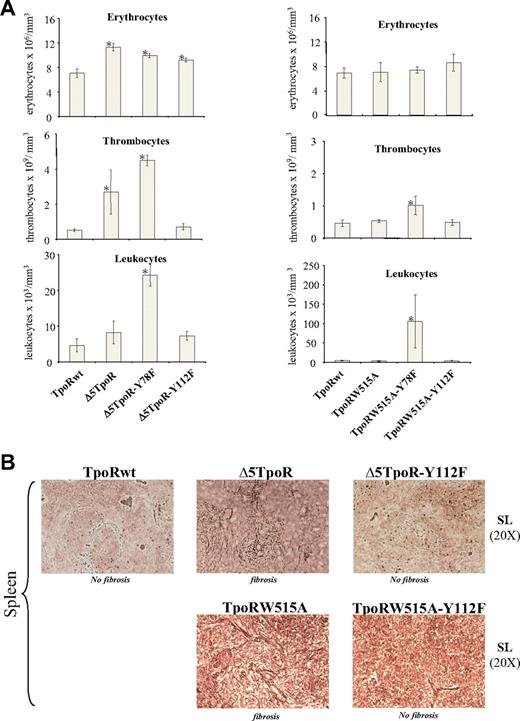
Comparing white blood cells and platelet counts 30 days after reconstitution (Figure 4A), we observed elevated levels in Δ5TpoR-Y78F mice compared with Δ5TpoR mice. These mice suffered from splenomegaly (data not shown) and harbored a predominant stimulation of the neutrophilic and megakaryocytic lineages in both bone marrow (supplemental Figure 2C) and spleen. Of note, erythroblastic precursors were more dominant in the spleen compared with the bone marrow (shown for Δ5TpoR-Y78F at day 60 after reconstitution, supplemental Figure 2A-B), with signs of ineffective erythropoiesis (intramedullary abortion, maturation block, erythrophagocytosis) and of dyserythropoiesis (supplemental Figure 2D). Erythrocyte numbers were only slightly increased, an effect we detected after 30 (Figure 4A) and 60 (data not shown) days of reconstitution. Interestingly, at 21 days after reconstitution, we detected granulocyte and megakaryocyte proliferation, along with stimulated erythropoiesis (without dyserythropoiesis), whereas at 45 days, a reticulinic diffuse myelofibrosis was detected in the marrow and spleen. Clearly, Δ5TpoR-Y78F induced a more severe myeloproliferative disorder than the Δ5TpoR with an increased number of thrombocytes and leukocytes (Figure 4A) and also enhanced spleen fibrosis (data not shown). This was not unexpected, as substitution of Y78 by phenylalanine is known to increase TpoR-mediated proliferation.18,19
In vivo effects of Δ5TpoR and TpoRW515A where cytosolic tyrosine residues were substituted to phenylalanine. (A) Peripheral blood counts of mice that received a transplant of bone marrow cells expressing the indicated Δ5TpoR (left) or TpoRW515 (right) mutants at day 30 after reconstitution. All mice present more than 95% GFP-positive cells at 3 weeks after reconstitution. (B) The Y112F mutation abrogates spleen myelofibrosis induced by Δ5TpoR or TpoRW515A at day 60 after reconstitution. Silver staining of spleen sections shows the presence of reticulin fibers in spleens from mice reconstituted with Δ5TpoR or TpoRW515A, but not in spleens from mice reconstituted with Δ5TpoR-Y112F, TpoRW515A-Y112F, or TpoRwt.
In vivo effects of Δ5TpoR and TpoRW515A where cytosolic tyrosine residues were substituted to phenylalanine. (A) Peripheral blood counts of mice that received a transplant of bone marrow cells expressing the indicated Δ5TpoR (left) or TpoRW515 (right) mutants at day 30 after reconstitution. All mice present more than 95% GFP-positive cells at 3 weeks after reconstitution. (B) The Y112F mutation abrogates spleen myelofibrosis induced by Δ5TpoR or TpoRW515A at day 60 after reconstitution. Silver staining of spleen sections shows the presence of reticulin fibers in spleens from mice reconstituted with Δ5TpoR or TpoRW515A, but not in spleens from mice reconstituted with Δ5TpoR-Y112F, TpoRW515A-Y112F, or TpoRwt.
Most importantly, the Y112F mutation abolished the myeloproliferative phenotype induced by Δ5TpoR (Figure 4A, day 30 after reconstitution; Figure 4B, day 60 after reconstitution), with spleens of relatively normal size (data not shown) and no myeloproliferation or fibrosis in the spleen (Figure 4B), suggesting that pathologic signaling leading to myelofibrosis induced by Δ5TpoR requires phosphorylation of Y112. The increase in erythrocyte numbers seen with Δ5TpoR (t test equal variance, P = .001) persisted for Δ5TpoR-Y112F at 30 (P = .008; Figure 4A) or 60 (P = .06; data not shown) days after reconstitution, but no myelofibrosis developed after 60 days for Δ5TpoR-Y112F (Figure 4B). Control mice reconstituted with any of the Y→F mutants in the TpoRwt background did not reconstitute faster than mice reconstituted with TpoRwt and did not show any signs of myeloproliferation. Expression levels of transduced TpoR were 2- to 2.5-fold higher than the endogenous TpoR, as revealed by Western blotting with an antibody directed to the murine TpoR, therefore detecting both transfected tagged receptor and endogenous receptor chains (data not shown). Furthermore, we tested in Ba/F3 cells the retroviruses used for reconstitution, and found them to have similar titers, which allowed us to sort equally infected Ba/F3 cells for similar GFP levels and assess surface levels of HA-tagged TpoR using flow cytometry with an anti-HA antibody (supplemental Figure 3B).
TpoRW515A mutants
A picture similar to that seen for Δ5TpoR Y→F mutants was noted when TpoRW515A and corresponding tyrosine mutants were used for bone marrow reconstitution assays. Just 30 days after reconstitution, when neither Δ5TpoR nor TpoRW515A reconstituted mice developed increased blood counts, the reconstituted mice with TpoRW515A-Y78F mutants began to exhibit a myeloproliferative disorder (Figure 4A right column) with splenomegaly (data not shown). When mice were examined at later times than day 45 after reconstitution, mutation of Y78 to phenylalanine led to a disease more severe than that of TpoRW515A, with marked spleen enlargement, hepatomegaly, and histology of advanced myelofibrosis in bone marrow and in spleen (data not shown). On the other hand, mutation of Y112 to phenylalanine in the context of the TpoRW515A prevented the development of the myeloproliferative disorder including the myelofibrosis, compared with the mice expressing the TpoRW515A (Figure 4B). Importantly, differences in the phenotypes were not related to different levels of expression of mutant TpoR, as Ba/F3 cells infected with the same viruses used for reconstitution showed similar cell surface HA-TpoR levels (supplemental Figure 3C).
Altogether these data indicate that (1) the Y112F mutation abolishes the MPD and myelofibrosis phenotype induced by Δ5TpoR or by TpoRW515A; and (2) the Y78F mutation enhances the MPD phenotype induced by Δ5TpoR or by TpoRW515A.
Role of cytosolic Y112 and Y78 in the induction of proliferation by TpoRW515A and Δ5TpoR
Ba/F3 cells are pro-B cells that are dependent on interleukin-3 for growth.43 Ectopic expression of the TpoR enables Ba/F3 cells to proliferate in response to Tpo. Ba/F3 cells were infected with bicistronic retroviruses encoding GFP as a marker and either the TpoRwt, Δ5TpoR TpoRW515A, or their Y→F mutants, as depicted in Figure 1B. Cell lines obtained after infection were sorted for equal GFP expression, washed, and grown in RPMI-10% fetal bovine serum in the absence of any cytokines, or in the presence of 3 ng/mL Tpo. As expected from previous reports, Ba/F3 cells expressing TpoR-Y78F grew faster in the presence of Tpo than cells expressing the TpoRwt, whereas cells expressing TpoR-Y112F grew slower after Tpo stimulation (Figure 5A). These data are in agreement with a requirement for Y112 for physiologic TpoR signaling.
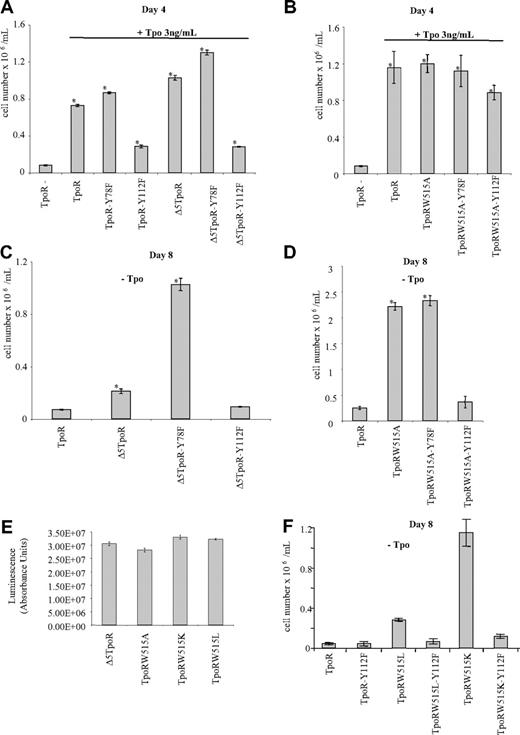
Role of TpoR cytosolic residues Y78 and Y112 in the proliferation induced in Ba/F3 cells by TpoR signaling. Proliferation of cells expressing either TpoRwt or the corresponding Y→F mutants, Δ5TpoR or the corresponding Y→F mutants (A), or TpoR W515A or the corresponding Y→F mutants (B) was examined after 4 days in the presence of 3 ng/mL Tpo. Shown are averages of triplicates ± SD of 1of 3 representative experiments. Proliferation of cells expressing Δ5TpoR (C) or TpoRW515A mutants (D) in the absence of any cytokine was examined after 8 days. Shown are averages of triplicates ± SD of 1 of 3 representative experiments. (E) Proliferation of Ba/F3 expressing either Δ5TpoR, TpoRW515A, TpoRW515K, or TpoRW515L in the absence of cytokine. Cell growth was measured using the Cell Titer Glo Kit (Promega). Shown are averages of absorbance units of triplicates ± SD obtained from 1 of 2 representative experiments. (F) The Y112F mutation inhibits proliferation induced in the absence of Tpo by TpoRW515L and TpoRW515K mutants. Shown are averages of cell counts performed in triplicates ± SD obtained from 1 of 2 representative experiments.
Role of TpoR cytosolic residues Y78 and Y112 in the proliferation induced in Ba/F3 cells by TpoR signaling. Proliferation of cells expressing either TpoRwt or the corresponding Y→F mutants, Δ5TpoR or the corresponding Y→F mutants (A), or TpoR W515A or the corresponding Y→F mutants (B) was examined after 4 days in the presence of 3 ng/mL Tpo. Shown are averages of triplicates ± SD of 1of 3 representative experiments. Proliferation of cells expressing Δ5TpoR (C) or TpoRW515A mutants (D) in the absence of any cytokine was examined after 8 days. Shown are averages of triplicates ± SD of 1 of 3 representative experiments. (E) Proliferation of Ba/F3 expressing either Δ5TpoR, TpoRW515A, TpoRW515K, or TpoRW515L in the absence of cytokine. Cell growth was measured using the Cell Titer Glo Kit (Promega). Shown are averages of absorbance units of triplicates ± SD obtained from 1 of 2 representative experiments. (F) The Y112F mutation inhibits proliferation induced in the absence of Tpo by TpoRW515L and TpoRW515K mutants. Shown are averages of cell counts performed in triplicates ± SD obtained from 1 of 2 representative experiments.
Expression of Δ5TpoR-Y78F increased the proliferation rate of Ba/F3 cells in response to Tpo compared with cells expressing the Δ5TpoR (Figure 5A). In contrast, cells expressing Δ5TpoR-Y112F grew significantly slower than cells expressing the Δ5TpoR. Similarly, cells expressing TpoRW515A-Y112F exhibited diminished growth compared with TpoRW515A (Figure 5B). These results support the notion that Y78 phosphorylation has a negative effect and Y112 phosphorylation has a positive effect on TpoR signaling.
Expression of the Δ5TpoR enables Ba/F3 cells to proliferate in the absence of any cytokine.25 Here we find that substitution of Y112 by phenylalanine abolishes the transforming ability of the Δ5TpoR in Ba/F3 cells, as cells expressing Δ5TpoR-Y112F died within a few days after cytokine removal (Figure 5C), whereas substitution of Y78 by phenylalanine increased the transforming ability of the Δ5TpoR in vitro. Introduction of the mutation Y112F completely disrupted autonomous growth induced by TpoRW515A (Figure 5D). Interestingly, Y78F mutation only weakly enhanced the autonomous growth of Ba/F3 cells induced by the TpoR W515A mutant. To compare the effects of TpoRW515K and TpoRW515L mutants to TpoRW515A and Δ5TpoR mutants, we performed proliferation assays on Ba/F3 cells expressing these mutants in cytokine-free medium. All these mutants were able to grow autonomously and exhibited similar growth rates (Figure 5E). As expected, the Y112F mutation also abolished the ability of TpoRW515L and TpoRW515K to induce autonomous growth (Figure 5F).
Role of cytosolic Y112 and Y78 in signaling by TpoR W515A and Δ5TpoR
Next we analyzed signaling induced by the various Y→F mutants of TpoRwt, Δ5TpoR, or TpoRW515A. We compared sorted cells that still required interleukin-3 for growth; expression levels of the different receptors are shown in Figure 6A. Cells were washed, starved for 5 hours, and subjected to 50 ng/mL Tpo for 15 minutes or not, and then activation of JAK2 and different signaling pathways was compared (Figure 6B-C).
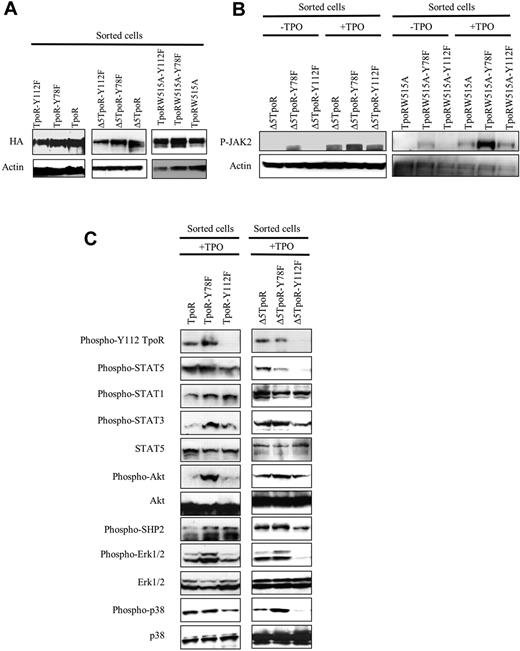
Effects of Y78F and Y112F mutations on signaling pathways activated by TpoR. Ba/F3 cells retrovirally transduced with Δ5TpoR or TpoRW515A and mutants thereof (Y78F or Y112F) were sorted for similar expression levels of GFP and examined for protein expression (A), activation of JAK2 (B), and activation of several downstream signaling pathways (C). Detection was performed by Western blotting with anti–phospho-specific antibodies that recognize the major phosphorylation sites that are linked to activation of catalysis (for kinases and phosphatases) or transcription (for STAT proteins). JAK2 activation was assessed by Western blotting with anti–phospho-JAK2 Y1007, as phosphorylation of activation loop Y1007 is obligatory for activation of JAK2 signaling. The Y78F mutation enhanced JAK2 activation and signaling via TpoRwt and Δ5TpoR. The Y112F mutation did not alter JAK2 activation, but inhibited downstream signaling, especially by Δ5TpoR.
Effects of Y78F and Y112F mutations on signaling pathways activated by TpoR. Ba/F3 cells retrovirally transduced with Δ5TpoR or TpoRW515A and mutants thereof (Y78F or Y112F) were sorted for similar expression levels of GFP and examined for protein expression (A), activation of JAK2 (B), and activation of several downstream signaling pathways (C). Detection was performed by Western blotting with anti–phospho-specific antibodies that recognize the major phosphorylation sites that are linked to activation of catalysis (for kinases and phosphatases) or transcription (for STAT proteins). JAK2 activation was assessed by Western blotting with anti–phospho-JAK2 Y1007, as phosphorylation of activation loop Y1007 is obligatory for activation of JAK2 signaling. The Y78F mutation enhanced JAK2 activation and signaling via TpoRwt and Δ5TpoR. The Y112F mutation did not alter JAK2 activation, but inhibited downstream signaling, especially by Δ5TpoR.
We assessed JAK2 activation, as revealed by acquisition of phosphorylation at activation loop Y1007 residue, which is absolutely required for activation of the kinase domain.44 The Y78F and Y112F mutations did not affect the JAK2 protein levels (not shown), but had significant effects on JAK2 activation (Figure 6B). Y78F mutation induces increased phosphorylation of JAK2 at Y1007 in Δ5TpoR and TpoRW515A (Figure 6B), indicating that Y78 actually prevents JAK2 activation. The Y78F exerts an enhancing effect on signaling by TpoRwt and TpoR mutants (Figure 6C), consistent with a positive effect on JAK2 activation, from which signaling is triggered.
The Y112F mutation did not inhibit activation of JAK2 (Figure 6B). We next assayed direct phosphorylation of Y112, using a phospho-Y112–specific antibody. Y112 phosphorylation was rapidly observed after TpoR activation and, as expected, it was absent in the Y112F mutants. To our knowledge, this is the first time phosphorylation of this residue is detected directly (see also Figure 3 and supplemental Table 1, mass spectrometry experiments indicating Y112 is directly phosphorylated). The Y112F mutation diminished levels of activated STAT5 and Erk1/2 in the TpoRwt (Figure 6C left), whereas for Δ5TpoR mutant STAT5, STAT3, Src homology 2 domain-containing protein, Erk1/2, and p38 MAP-kinases were all more significantly inhibited (Figure 6C right).
Taken together, these data indicate that Y78 negatively modulated TpoR, which is consistent with a key role of this tyrosine residue in down-modulation of TpoR signaling.13,19 Y78F mutation enhanced signaling via MAP-kinase pathways such as Erk1/2 and p38, which may contribute to the pathogenicity of the Δ5TpoR and W515A constitutively active mutants in vivo. We show that the negative effect of Y78 involves an inhibition of activation of JAK2, which is manifest also in the absence of Tpo, as detected for the strong TpoRW515A mutant (Figure 6B). In contrast, Y112 promotes downstream signaling of TpoR and TpoR mutants, but not JAK2 activation; when effects of Y112F are compared in TpoRwt and Δ5TpoR, it appears that effects are more pronounced and involve most pathways of the latter. These data are consistent with the in vivo experiments where, for both Δ5TpoR and TpoRW515A, the mutation of Y112 to phenylalanine abolished phenotype.
Role of signaling pathways on the transformation ability of the active TpoR mutants
To also test signaling in reconstituted mice, we snap-froze spleens from mice at day 60 after reconstitution and then performed Western blot analysis to assess for activation of STAT5, STAT3, MAP-kinase, Erk1/2, and Akt. As spleens contain several cell types, our analysis is only an approximate and average assessment of the state of activation of the different signaling pathways. Supplemental Figure 4 shows that TpoRW515A spleens exhibited general activation of the STAT, MAP-kinase, and Akt, and that the Y112F mutation decreased mainly the MAP-kinase Erk1/2 activation, and only to a lower degree activation of the other pathways. Levels of HA-TpoR were higher in the TpoRW515A spleens, consistent with the splenomegaly due to proliferation of TpoRW515A-expressing cells. Nevertheless, even if normalization is performed for amount of HA-TpoR (and not like in supplemental Figure 4 for protein levels), the decrease in Erk1/2 levels of activation remains significant.
The immunoprofiling assay performed in Ba/F3 cell lines pointed out that MAP-kinase proteins such as Erk1/2 or p38 appear to be overactivated in cells expressing TpoR constitutively active mutants (supplemental Table 1, supplemental Figure 5). However, these data reflect number of times a particular tyrosine-phosphorylated peptide is detected in the dataset and are not a stringent quantitative assessment. We tested the effects of several inhibitors on the proliferation of Ba/F3 cell lines expressing TpoRW515A and Δ5TpoR, which were growing autonomously. Mitogen-activated protein kinase kinase 1/2 (MEK1/2) inhibitor (UO126) blocked the proliferation of these cells, but the JNK1/2/3 inhibitor SP600125 or the p38 inhibitor SB202190 exerted no inhibition (supplemental Figure 5). In addition to the MEK inhibitor UO126, the JAK2 inhibitor AG490 and the PI-3-kinase inhibitor Ly294002 also blocked cell proliferation. Taken together, our data suggest that phosphorylated Y112 might mediate the pathogenic signaling by TpoRW515A and Δ5TpoR possibly through the activation of MAP-kinase pathways, but also via the other pathways, the PI-3-kinase and STAT pathways.
Discussion
Our major observation is that cytosolic Y112 of TpoR is absolutely required for mediating the pathologic effects of TpoRW515 mutants, both in cell lines and in vivo in bone marrow adoptive transfer experiments. Both TpoRW515A and a TpoR mutant where the entire K/RWQFP motif was deleted (Δ5TpoR) induced in vivo, in bone marrow adoptive transfer experiments, a myeloproliferative phenotype with myelofibrosis. We identify the W515A mutation in 2 myelofibrosis patients. These results, along with the similar phenotype induced by the TpoRW515L and W515K mutants,31,32 indicate that the loss of W515, and not the acquisition of a particular residue is responsible for pathologic signaling. This is also supported by another recent study that described a patient with the W515A mutation.45
Although several previous studies examined the effects of Y→F mutations in the TpoR cytosolic tyrosine residues, our data are the first to show that cytosolic TpoR Y112 and Y78 are phosphorylated in living cells transformed by TpoRW515A. Examination of signaling by TpoRwt and mutant TpoRs with Y→F substitution indicated that the Y112F mutation significantly decreases signaling of Δ5TpoR and TpoRW515A via STAT5, STAT3, Akt, and MAP-kinase, the latter being strongly activated in transformed cells, and that Y78 plays a strong negative role in Δ5TpoR and TpoRW515A signaling. Y78 and Y112 exert negative and positive effects on the activity of TpoRW515 mutants, respectively, and the mechanisms of their effects are also different. Y112 does not affect JAK2 activation, but promotes downstream signaling, as expected, because in its phosphorylated form, Y112 will attract SH2-containing signaling molecules, such as Shc. In contrast, Y78 prevents JAK2 activation and, consequently, the Y78F mutation would activate general TpoR signaling. Of note, the Y78F mutation introduced in the context of the TpoRwt is insufficient to induce pathology in mice, thus Y78 functions to limit receptor function either after ligand-induced activation or in the context of a mutated TpoR. Interestingly, Y78 was proposed to bind negative regulatory molecules or trigger receptor down-modulation and internalization.46 In our experiments, we detected similar surface levels of TpoR mutants (supplemental Figure 3), but we did not perform detailed kinetics of internalization and recycling. Y78 exhibited the same action on TpoRwt and on Δ5TpoR or TpoRW515A mutants, suggesting that the conformational change induced by W515 mutants does not affect the mechanisms by which Y78 down-modulates receptor signaling, and vice versa that Y78 cannot prevent the conformational change induced by W515 mutations, and this is directly reflected in regulation of JAK2 activation (model in Figure 7).
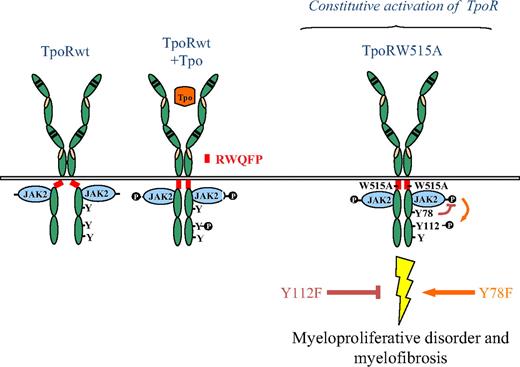
Model for the roles of TpoR Y112 and Y78 in pathologic signaling by TpoR W515 mutants. The wild-type TpoR (TpoRwt) contains a juxtamembrane domain K(R)WQFP that maintains the unliganded receptor inactive. TpoRwt is activated upon Tpo stimulation leading to conformational change of the receptor and to activation of major signaling pathways. In the absence of the K(R)WQFP juxtamembrane motif (Δ5TpoR), or in situations where the W515 of this motif is substituted by other residues (ie, W515A), the receptor assumes an active dimeric conformation, which activates JAK2 in a ligand-independent manner. Y78 plays a negative role in signaling through TpoRwt or the active TpoR. Mutation of Y78 into phenylalanine in the context of the activating W515 mutations led to strong pathologic signaling and myelofibrosis. In contrast, the TpoR-Y112F mutation decreases signaling of the receptor and abolishes the myeloproliferative and myelofibrosis phenotype.
Model for the roles of TpoR Y112 and Y78 in pathologic signaling by TpoR W515 mutants. The wild-type TpoR (TpoRwt) contains a juxtamembrane domain K(R)WQFP that maintains the unliganded receptor inactive. TpoRwt is activated upon Tpo stimulation leading to conformational change of the receptor and to activation of major signaling pathways. In the absence of the K(R)WQFP juxtamembrane motif (Δ5TpoR), or in situations where the W515 of this motif is substituted by other residues (ie, W515A), the receptor assumes an active dimeric conformation, which activates JAK2 in a ligand-independent manner. Y78 plays a negative role in signaling through TpoRwt or the active TpoR. Mutation of Y78 into phenylalanine in the context of the activating W515 mutations led to strong pathologic signaling and myelofibrosis. In contrast, the TpoR-Y112F mutation decreases signaling of the receptor and abolishes the myeloproliferative and myelofibrosis phenotype.
No crystal or NMR structure exists for any of the 30 cytokine receptors or their cognate JAKs. We and others have argued for rigidity at least for the initial segments of cytokine receptor cytosolic domains,17,27,28 despite a rather flexible/unstructured nature of the entire cytosolic domain.29 TpoR might contain both rigid (juxtamembrane) and flexible (distal sequences with tyrosines that become phosphorylated) regions in its cytosolic domain. In that respect, almost 40% of the human proteome might be intrinsically disordered or not folded in the absence of a partner.47,48 Cytokine receptors are coupled intracellularly to Janus kinases with which they form tight functional complexes.14,30,49,50 This suggests that the conformation of the receptors' cytosolic domains might depend on interaction with JAKs, and also that the ability of the kinase domains of Janus kinases to phosphorylate receptor tyrosine residues might depend on receptor conformation. Our findings indicate that a change at the outset of the cytosolic domain (W515 is the second cytosolic residue) induces receptor activation and a conformational change 100 amino acids downstream (around Y112) with clear consequences for signaling.
Ba/F3 cells transformed to autonomous growth by TpoRW515 mutants exhibit enhanced MAP-kinase activation, the Y112F mutation leads to reduced Erk1/2 activation in spleens of reconstituted mice (supplemental Figure 4), and an inhibitor of the upstream kinase Erk1/2 kinase (or MEK) inhibits proliferation of Ba/F3 cells expressing TpoRW515A (supplemental Figure 5). However, inhibitors of PI-3-kinase also reduce proliferation, and the Y112F mutation also reduces STAT activation, suggesting that a combination of signaling molecules might mediate the oncogenic effect of TpoRW515 mutants. The MAP-kinase pathway has previously been linked to signaling via cytosolic TpoR Y112,18-21 and we hypothesize that it might synergize with other Y112 activated pathways and may be important for MPNs induced by TpoR mutants, although residual MAP-kinase activation can be detected in receptor truncations that lack the distal 50 amino acid residues, which includes Y112.19 It is possible that phosphorylated Y112 in TpoRW515 mutants specifically assembles a signaling complex that induces megakaryocyte proliferation and that is stabilized due to the W515 mutations in the upstream juxtamembrane domain.
Our results also suggest that expression of the Δ5TpoR or TpoRW515A in the HSC compartment, which has recently been documented,51 preferentially shifts differentiation toward the megakaryocytic and granulocytic lineages. This hypothesis will need to be confirmed by long-term and short-term colony assays on bone marrow cells. A skewing of HSCs toward erythroid differentiation was reported for JAK2V617F in polycythemia vera.52 The in vivo phenotype induced by JAK2V617F is much milder37,53-56 than that induced by TpoRW515L31 or by Δ5TpoR/TpoRW515A mutants (Figures 2,Figure 3–4). We can state that in the C57Bl6 mice, side-by-side reconstitution with JAK2V617F and TpoRW515 mutants reveals a much more rapid and severe myeloproliferative disease induced by TpoRW515 mutants. Previous such experiments were reported for TpoRW515L only in Balb/c mice.31 Several other constitutively active TpoR (Mpl) mutants have been described, such as the transmembrane S505N mutant,57 in familial and sporadic essential thrombocytosis58 and the T487A mutant in a non-Down syndrome childhood acute megakaryoblastic leukemia; this mutant induces a myeloproliferative disorder in mouse bone marrow reconstitution assays.59 It will be interesting to test the role of Y112 in inducing myeloid malignancies by these transmembrane and juxtamembrane TpoR mutants.
The discovery of JAK2V617F prompted extensive efforts toward identifying inhibitors of JAK2 to be used in the treatment of MPNs. Several adenosine triphosphate competitive inhibitors have been described60-63 that show different degrees of selectivity with respect to JAK2. These molecules do not discriminate between JAK2V617F and the wild-type JAK2, but can alleviate the phenotype induced by JAK2V617F.64,65 Inhibition of wild-type JAK2 is predicted to induce myelosuppressive effects, with anemia and thrombocytopenia. It remains to be seen whether in MPN patients, especially those with myelofibrosis, such inhibitors would be able to reduce allele burden and reverse bone marrow fibrosis. Our present results indicate that for the TpoRW515 mutants, phosphorylation of Y112 is crucial for developing the myelofibrosis phenotype. This is consistent with several lines of evidence that supported a major role for megakaryocyte proliferation and excessive TpoR signaling in the establishment of myelofibrosis.66,67 Moreover, TpoR Y112 might also be crucial for myelofibrosis in patients with the JAK2V617F mutation. We suggest that the TpoR region around Y112 might be a good target for pharmacologic inhibition for preventing pathologic signaling that leads to myelofibrosis. A small molecule blocking phosphorylated Y112 is not expected to affect steady-state megakaryocyte differentiation and platelet formation because the distal 60 amino acids of TpoR are not absolutely essential for steady-state megakaryocytopoiesis, as demonstrated by knock-in studies.34
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Ivan Théate and Jean-Philippe Defour for histopathology diagnosis, André Tonon for cell sorting, Guy Warnier for mouse breeding, and Céline Mouton for expert technical support.
This work was supported by the FRS (Fonds de la Recherche Scientifique)–FNRS, the Salus Sanguinis Foundation, the Action de Recherche Concertée MEXP31C1 of the University Catholique de Louvain, the Fondation contre le Cancer, the Pôle d'attraction interuniversitaire (PAI) Program BCHM61B5, and the Atlantic Philanthropies. S.N.C. is Research Associate of the FRS-FNRS.
Authorship
Contribution: C.P., J.S., and S.N.C. designed research, performed experiments, and wrote the paper; R.C., V.G., K.A.L., X.Z., J.R., J.V.H., W.V., and S.G. performed research and wrote the paper; H.A.P. and J.-M.S. analyzed pathology samples and wrote the paper; and R.D.P. analyzed research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Stefan N. Constantinescu, Signal Transduction and Molecular Hematology Laboratory, Ludwig Institute for Cancer Research Ltd, de Duve Institute, Université Catholique de Louvain, Avenue Hippocrate 74, UCL 75-4, Brussels B-1200, Belgium; e-mail: stefan.constantinescu@bru.licr.org; or Judith Staerk, Whitehead Institute for Biomedical Research, 9 Cambridge Center, Cambridge, MA 02142; e-mail: staerk@wi.mit.edu.
References
Author notes
C.P. and J.S. contributed equally to this study.