To the editor:
Koljonen et al described an association between chronic lymphocytic leukemia (CLL) and Merkel cell polyomavirus (MCV)–positive Merkel cell carcinoma (MCC) in a recent large population-based survey from Finland.1 CLL has been reported as a tumor significantly associated with MCC, validating the general findings of this study.2,3 This association suggests the possibility that MCV may play a role in CLL as well as in MCC.
MCV, initially found in MCC by digital transcriptome subtraction, has subsequently been confirmed to be clonally integrated in approximately 80% of MCC tumors.4 Mutations in MCV large-T antigens sequenced from MCC tumors show tumor-specific truncation signatures that ablate viral replication functions of this protein while retaining domain interactions important for polyomavirus-induced carcinogenesis.5 MCV is a common human infection, but MCC patients with MCV-infected tumors have significantly elevated anti-MCV IgG responses.6,7
We previously examined 33 CLL tissues by qPCR and 12 by MCV large-T antigen immunostaining.8 All specimens were negative for MCV genome or protein expression, however, none of these cases were associated with a history of MCC. Although the occurrence of these 2 neoplasms are significantly associated, the uncommon nature of MCC has resulted in only rare published cases.9,10 In this report, we present 2 cases of concurrent CLL and MCV. We specifically sought evidence of MCV T antigen protein expression in CLL cells to circumvent the extremely high sensitivity of PCR, which can detect incidentally infected cells in tissues.
Case 1.
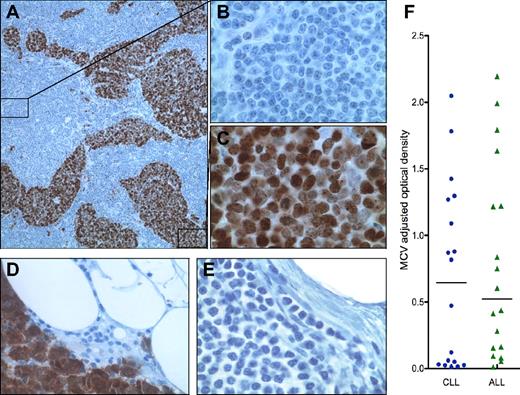
A 74-year-old female with CLL presented with a right thigh mass and enlarged right groin mass. Pathologic examination of thigh mass reveals immunohistochemically confirmed MCC. The groin resection contained metastatic MCC to lymph nodes with CLL. Examination of a lymph node by CM2B4 and CD20 immunohistochemical staining shows MCV T antigen protein expression only in the metastatic MCC (Figure 1A,C) but not in CD20-positive CLL cells (Figure 1B).
(A) Case 1: lymph node with both CLL (10×/0.30 NA; B) and metastatic MCC (100×/1.40 NA oil objective; C) immunohistochemically stained (Dako EnVision+ Kit) with CM2B4,8 a mouse monoclonal antibody specific to MCV T antigen; and counterstained with hematoxylin. Case 2: MCC metastatic to lymph node showing strong nuclear expression of MCV T antigen expression (40×/0.75 NA; D) and CLL (40×/0.75 NA; E) demonstrating effacement of normal nodal architecture with a population of cells comprised predominantly of small lymphocytes with scant cytoplasm, round nuclei, and clumped chromatin. Images were acquired on an Olympus Provis AX70 using Spot 3.5.9 software for Mac OS (Diagnostic Instruments). All images were processed using Adobe Photoshop CS3. (F) Anti-MCV antibody levels (colored dots and triangles), as measured with MCV virus-like particle enzyme immunoassay (VLP EIA), are not significantly different in sera from CLL and ALL patients. Median MCV optical density values are indicated by horizontal lines, with bars representing interquartile values.
(A) Case 1: lymph node with both CLL (10×/0.30 NA; B) and metastatic MCC (100×/1.40 NA oil objective; C) immunohistochemically stained (Dako EnVision+ Kit) with CM2B4,8 a mouse monoclonal antibody specific to MCV T antigen; and counterstained with hematoxylin. Case 2: MCC metastatic to lymph node showing strong nuclear expression of MCV T antigen expression (40×/0.75 NA; D) and CLL (40×/0.75 NA; E) demonstrating effacement of normal nodal architecture with a population of cells comprised predominantly of small lymphocytes with scant cytoplasm, round nuclei, and clumped chromatin. Images were acquired on an Olympus Provis AX70 using Spot 3.5.9 software for Mac OS (Diagnostic Instruments). All images were processed using Adobe Photoshop CS3. (F) Anti-MCV antibody levels (colored dots and triangles), as measured with MCV virus-like particle enzyme immunoassay (VLP EIA), are not significantly different in sera from CLL and ALL patients. Median MCV optical density values are indicated by horizontal lines, with bars representing interquartile values.
Case 2.
A 66-year-old male diagnosed with CLL 12 years earlier presented with a left forearm bump (diagnosed as MCC). Biopsy and left axillary dissection reveals cytokeratin-20–positive MCC metastatic to 9 of 80 lymph nodes, virtually all having effacement of normal architecture by CLL. A node containing metastatic MCC illustrates robust nuclear expression of MCV T antigen in tumor cells (Figure 1D), whereas the CLL component is negative (Figure 1E).
We also collected 18 CLL and 18 acute lymphoblastic leukemia (ALL) age-matched sera. Ten of 18 (55.6%) CLL and 12 of 18 (66.7%) ALL patients showed serologic evidence for MCV exposure (Figure 1F). The median MCV IgG titer for the CLL sera (0.645 OD units) was similar to, and not statistically different from, ALL patients (0.521 OD units).
These findings, together with our previous study,8 indicate that MCV is not likely to be directly involved in chronic lymphocytic leukemogenesis. We also do not find MCV infection more commonly among CLL patients than among a representative control group.6,7 Other possible explanations for the association between these 2 cancers include CLL-related immunosuppression predisposing to MCC or a pathologic immune response to MCV infection in persons predisposed to CLL.
Authorship
Acknowledgments: We thank Dr Michael Keating for providing clinical information. This study was supported in part by awards from the Al Copeland Foundation and by National Institutes of Health grants CA136363 and CA120726. Y.C. and P.S.M. are funded as American Cancer Society Research Professors.
Contribution: Y.L.T., R.A., and S.C.S. performed the research; Y.L.T., R.A., S.C.S., K.B., P.M.C., Y.C., and P.S.M. analyzed and interpreted the data; and Y.L.T., R.A., Y.C., and P.S.M. wrote the manuscript.
Conflict-of-interest disclosure: The University of Pittsburgh has commercially licensed the CM2B4 antibody.
Correspondence: Patrick S. Moore, University of Pittsburgh, 5117 Centre Ave, Pittsburgh, PA 15213; e-mail: psm9@pitt.edu.
References
National Institutes of Health
Author notes
Y.C. and P.S.M. contributed equally to this letter.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal