Abstract
MicroRNAs are endogenously expressed small noncoding RNAs that regulate gene expression on the posttranscriptional level. The miR-17-92 cluster (encoding miR-17, -18a, -19a/b, -20a, and miR-92a) is highly expressed in tumor cells and is up-regulated by ischemia. Whereas miR-92a was recently identified as negative regulator of angiogenesis, the specific functions of the other members of the cluster are less clear. Here we demonstrate that overexpression of miR-17, -18a, -19a, and -20a significantly inhibited 3-dimensional spheroid sprouting in vitro, whereas inhibition of miR-17, -18a, and -20a augmented endothelial cell sprout formation. Inhibition of miR-17 and miR-20a in vivo using antagomirs significantly increased the number of perfused vessels in Matrigel plugs, whereas antagomirs that specifically target miR-18a and miR-19a were less effective. However, systemic inhibition of miR-17/20 did not affect tumor angiogenesis. Further mechanistic studies showed that miR-17/20 targets several proangiogenic genes. Specifically, Janus kinase 1 was shown to be a direct target of miR-17. In summary, we show that miR-17/20 exhibit a cell-intrinsic antiangiogenic activity in endothelial cells. Inhibition of miR-17/20 specifically augmented neovascularization of Matrigel plugs but did not affect tumor angiogenesis indicating a context-dependent regulation of angiogenesis by miR-17/20 in vivo.
Introduction
Recent studies describe a fundamental role of microRNAs (miRs) in development and diseases.1-4 MiRs are small 18- to 24-nucleotide single-stranded noncoding RNAs that regulate mRNA degradation and translation.5 Together with a protein complex known as RNA-induced silencing complex, miRs bind to sites in the 3′-untranslated region (3′-UTR) and induce degradation or reduce the translation of the targeted mRNA. Approximately 700 miRs have been identified in humans. Each miR can regulate up to several hundreds of targets, and it is considered that translation of approximately one-third of proteins is regulated by miRs.6
First evidence that miRs control vessel formation was obtained by depletion of the RNA endonuclease Dicer, which mediates miR maturation. Mice deficient for endothelial Dicer showed an aberrant vessel growth,7 and silencing of Dicer in endothelial cells (ECs) reduced in vitro angiogenesis.8,9 Several specific miRs were shown to control angiogenesis, such as miR-126, which is highly expressed in ECs and is essential for blood vessel growth in zebrafish10 and mice.11,12 In addition, miR-221 and miR-222 block angiogenesis,13 whereas miR-27b,8 miR-130a,14 and members of the let-7 family8,15 increase angiogenesis.
The miR-17-92 cluster was among the first miRs that were linked to tumor angiogenesis. The miR-17-92 cluster is a typical example of a polycistronic miR cluster encoding the miRs miR-17, miR-18a, miR-19a/b, miR-20a, and miR-92a, which are highly expressed in several tumors.16 Particularly, miR-17 and miR-20a were shown to control cellular proliferation and apoptosis by targeting the E2F family of transcription factors17,18 and by down-regulating the tumor suppressor p2119,20 and the proapoptotic protein BIM.20 Overexpression of the entire miR-17-92 cluster in myc-induced tumors increased angiogenesis by a paracrine mechanism.21 This proangiogenic function has been attributed to the down-regulation of the antiangiogenic molecules thrombospondin-1 (TSP-1) and connective tissue growth factor (CTGF), which are targeted by miR-18 and miR-19.21 Combined overexpression of miR-17, miR-18a, and miR-20a also partially rescued the impaired endothelial network formation induced by silencing of Dicer in vitro.7 Injection of miR-17 in combination with let-7b into the ovaries of Dicer-deficient mice partially normalized corpus luteum angiogenesis.15 In contrast, overexpression of miR-92a suppressed angiogenic sprout formation in vitro and interfered with intersegmental vessel growth in zebrafish.22 Vice versa, inhibition of miR-92a in vivo using antagomirs augmented neovascularization and functional recovery after ischemia.22
Here, we investigated the role of individual members of the miR-17-92 cluster for the cell-intrinsic angiogenic activity of ECs and determined the effects on neovascularization in vivo. Surprisingly, overexpression of the individual members of the miR-17-92 cluster, namely, miR-17, miR-18a, miR-19a, and miR-20a, reduced EC sprouting, whereas inhibitors of these miRs augmented angiogenesis in vitro, indicating that the miR-17-92 cluster provides a cell-intrinsic antiangiogenic activity in ECs. Combined inhibition of miR-17 and miR-20a by antagomir treatment in vivo additionally enhanced angiogenic sprouting, but did not affect tumor angiogenesis.
Methods
Protocols are available online. The animal experiments were conducted according to the principles of laboratory animal care as well as according to the German national laws. The studies have been approved by the local ethical committee (Regierungspräsidium Darmstadt). All microarray data have been deposited into the Gene Express Omnibus public database (National Center for Biotechnology Information) under accession number GSE20745.
Results
Individual members of the miR-17-92 cluster inhibit angiogenesis in vitro
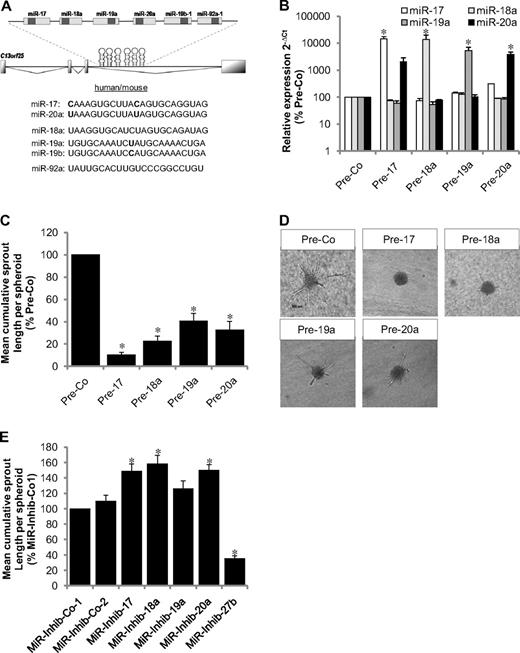
To investigate the cell-intrinsic function of the miR-17-92 cluster in ECs, precursors of the individual members miR-17, miR-18a, miR-19a, and miR-20a were expressed in ECs (Figure 1A) to study the effect on angiogenesis in vitro. Transfection of human umbilical vein endothelial cells (HUVECs) with precursors of miR-18a, miR-19a, and miR-20a specifically increased the expression of the mature miR (Figure 1B), whereas mature miR-17 and miR-20a were detected when overexpressing the precursor of miR-17 (Figure 1B). Overexpression of all members of the miR-17-92 cluster significantly inhibited EC sprouting in a 3-dimensional spheroid model (Figure 1C-D). Likewise, vascular network formation in Matrigel models and EC migration were blocked by overexpressing the individual members of the miR-17-92 cluster (supplemental Figure 1A-B, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). To exclude unspecific effects of the overexpressed precursors, we used a precursor for miR-27b, which did not inhibit angiogenic sprouting (data not shown), and confirmed the antiangiogenic activity of miR-17 using miR mimetics (supplemental Figure 1C).
Effect of the members of the miR-17-92 cluster on angiogenesis in human ECs. (A) Schematic illustration of the miR-17-92 cluster and sequences of the individual members. (B) HUVECs were transfected with miR precursors or a control precursor (Pre-Co) as indicated, and expression of mature miRs was detected by stem loop PCR after 24 hours. Data were normalized to RNU48; n = 3. (C-D) Inhibition of sprout formation in the spheroid assay (n = 10 spheroids/experiment; n = 5-11 experiments). *P < .05 versus Pre-Co. (D) Representative images. Scale bar represents 100 μm. (E) HUVECs were transfected with miR inhibitiors as indicated, and sprout length of spheroids was quantified (n = 10 spheroids/experiment; n = 6 experiments). *P < .05 versus MiR-Inhib-Co-2.
Effect of the members of the miR-17-92 cluster on angiogenesis in human ECs. (A) Schematic illustration of the miR-17-92 cluster and sequences of the individual members. (B) HUVECs were transfected with miR precursors or a control precursor (Pre-Co) as indicated, and expression of mature miRs was detected by stem loop PCR after 24 hours. Data were normalized to RNU48; n = 3. (C-D) Inhibition of sprout formation in the spheroid assay (n = 10 spheroids/experiment; n = 5-11 experiments). *P < .05 versus Pre-Co. (D) Representative images. Scale bar represents 100 μm. (E) HUVECs were transfected with miR inhibitiors as indicated, and sprout length of spheroids was quantified (n = 10 spheroids/experiment; n = 6 experiments). *P < .05 versus MiR-Inhib-Co-2.
To determine whether inhibition of the endogenously expressed members of the miR-17-92 cluster is sufficient to induce angiogenic sprouting, we inhibited the individual miRs using hairpin inhibitors. Inhibition of miR-17, miR-18a, and miR-20a increased spheroid sprouting approximately 1.5-fold, whereas miR-19a only slightly enhanced angiogenesis in vitro (Figure 1E). As a control, we showed that inhibition of miR-27b blocks angiogenic sprouting as previously described.8 To assess the in vivo relevance of these findings, the individual members of the miR-17-92 cluster were specifically inhibited by antagomir23 treatment and neovascularization was determined using the Matrigel plug assay. First, we confirmed the specific knockdown of the targeted miR in antagomir-treated mice (Figure 2A-B; supplemental Figure 2A). Only antagomir-17 exhibited an off-target effect on the expression of the closely related miR-20a as detected by 2 different probe-based quantitative polymerase chain reaction (PCR) methods (Figure 2A-B). These data indicate that the antagomir designed to suppress miR-17 also inhibits miR-20a because of large overlaps in the sequence (differs only in 2 nucleotides), a finding that is consistent with previous studies using LNA-miR-17 inhibitors.24 Therefore, this antagomir is referred to as antagomir-17/20 from here on. Three injections of antagomir-17/20 (8 mg/kg body weight each) at days 0, 2, and 4 after subcutaneous implantation of Matrigel increased the number of perfused vessels that invaded the Matrigel plug in vivo (Figure 2C-D), whereas specific blockade of miR-18a, miR-19a, or mir-20a showed a trend but no significant effect on neovascularization (Figure 2C). Similar effects were seen when antagomirs were injected only once at day 1 after implantation of the plugs (supplemental Figure 2B).
Inhibition of miR-17 enhances angiogenesis in mice. (A-B) Effect of systemic infusion of 3 intravenous injections of antagomirs targeting miR-17, miR-18a, miR-19a, and miR-20a (8 mg/kg body weight, n = 3-5 mice per group, 2 plugs/mouse) or a control antagomir (antagomir-Co, n = 6) on miR expression in hearts harvested 7 days after the first injection. MiR expression was detected by a real-time PCR method using a universal TaqMan probe in combination with a miR-specific forward primer (A) and a TaqMan miRNA assay with higher specificity (B). (C-D) Effect of antagomir intravenous infusion at days 0, 2, and 4 on the number of lectin-perfused vessels in Matrigel plugs in vivo after 7 days; n = 4-10 per group. *P < .05 versus Antagomir-Co. (D) Representative images. Scale bars represent 20 μm.
Inhibition of miR-17 enhances angiogenesis in mice. (A-B) Effect of systemic infusion of 3 intravenous injections of antagomirs targeting miR-17, miR-18a, miR-19a, and miR-20a (8 mg/kg body weight, n = 3-5 mice per group, 2 plugs/mouse) or a control antagomir (antagomir-Co, n = 6) on miR expression in hearts harvested 7 days after the first injection. MiR expression was detected by a real-time PCR method using a universal TaqMan probe in combination with a miR-specific forward primer (A) and a TaqMan miRNA assay with higher specificity (B). (C-D) Effect of antagomir intravenous infusion at days 0, 2, and 4 on the number of lectin-perfused vessels in Matrigel plugs in vivo after 7 days; n = 4-10 per group. *P < .05 versus Antagomir-Co. (D) Representative images. Scale bars represent 20 μm.
In summary, these data indicate that overexpression of the individual members of the miR-17-92 cluster blocks angiogenesis in vitro, whereas particularly the combined inhibition of miR-17 and miR-20a promotes angiogenesis in vitro and in vivo.
Regulation of tumor angiogenesis
Previous studies suggested that overexpression of the miR-17-92 cluster in tumor cells enhances tumor angiogenesis.21 The proangiogenic activity in this model has been attributed to the suppression of antiangiogenic factors by tumor cells, which might act in a paracrine manner on ECs. Therefore, we compared the angiogenic activity of conditioned medium of tumor cells, which had been transfected with the precursor molecules for the individual members of the miR-17-92 cluster, with that of likewise transfected ECs (Figure 3A-B). Indeed, conditioned medium of LLC1 tumor cells overexpressing miR-17, miR-19a, and miR-20a (supplemental Figure 3) slightly enhanced angiogenic sprouting of ECs (Figure 3B). In contrast, conditioned medium derived from ECs transfected with miR-17, miR-18a, or miR-19a showed a trend toward reduction of angiogenic activity of ECs (Figure 3B). To determine the net effect on tumor growth in vivo, we tested the effect of antagomir-17/20 in a Lewis lung carcinoma tumor model. Injection of antagomir-17/20 induced a minor nonsignificant increase in tumor size, volume, and weight (Figure 3C-D); however, tumor vascularization, as measured by counting endomucin-stained capillaries, was not increased (Figure 3E). These data were confirmed by detecting in vivo perfused lectin-positive vessels in the tumor sections showing that antagomir-17/20 treatment did not increase the perfusion of the implanted tumors (Figure 3F). Moreover, increasing the number of antagomir-17/20 injections did not affect tumor growth despite a sufficient inhibition of miR-17 expression in explanted tumors (supplemental Figure 4). Overall, these data suggest that antagomir-17/20 selectively enhances neovascularization of Matrigel plugs but does not affect tumor angiogenesis.
Effect of members of the miR-17-92 cluster on paracrine activity of tumor cells in vitro and tumor angiogenesis in vivo. (A-B) HUVECs or LLC1 tumor cells were transfected with the respective precursors. Medium was changed to Dulbecco modified Eagle medium with 0.05% bovine serum albumin after 1 day. Conditioned medium was collected at day 2, and 10 times concentrates were transferred to collagen-embedded spheroids with nontransfected HUVECs as illustrated in panel A. Quantification of spheroid sprout length was performed after incubation of the spheroids with the conditioned medium for 24 hours; n = 4. (C-D) LLC1 tumor cells were subcutaneously injected in mice. Antagomir-17/20 (8 mg/kg body weight) was intravenously injected once as indicated. Tumor size was measured daily (C), and tumor volume and weight were detected in explanted tumors (D) at day 13; n = 7 for Antagomir-Co and n = 6 for Antagomir-17. (E) Tumor angiogenesis was detected in sections stained with the endothelial marker endomucin. A secondary antibody conjugated to Alexa Fluor 555 was used. The number of vessels was counted manually; n = 7 for Antagomir-Co and n = 6 for Antagomir-17. (F) Perfused vessels were detected by intravenous infusion of fluorescein isothiocyanate-conjugated lectin and were quantified by automatic measurement of the pixel region in each section; n = 7 for Antagomir-Co and n = 6 for Antagomir-17. Scale bars represent 20 μm.
Effect of members of the miR-17-92 cluster on paracrine activity of tumor cells in vitro and tumor angiogenesis in vivo. (A-B) HUVECs or LLC1 tumor cells were transfected with the respective precursors. Medium was changed to Dulbecco modified Eagle medium with 0.05% bovine serum albumin after 1 day. Conditioned medium was collected at day 2, and 10 times concentrates were transferred to collagen-embedded spheroids with nontransfected HUVECs as illustrated in panel A. Quantification of spheroid sprout length was performed after incubation of the spheroids with the conditioned medium for 24 hours; n = 4. (C-D) LLC1 tumor cells were subcutaneously injected in mice. Antagomir-17/20 (8 mg/kg body weight) was intravenously injected once as indicated. Tumor size was measured daily (C), and tumor volume and weight were detected in explanted tumors (D) at day 13; n = 7 for Antagomir-Co and n = 6 for Antagomir-17. (E) Tumor angiogenesis was detected in sections stained with the endothelial marker endomucin. A secondary antibody conjugated to Alexa Fluor 555 was used. The number of vessels was counted manually; n = 7 for Antagomir-Co and n = 6 for Antagomir-17. (F) Perfused vessels were detected by intravenous infusion of fluorescein isothiocyanate-conjugated lectin and were quantified by automatic measurement of the pixel region in each section; n = 7 for Antagomir-Co and n = 6 for Antagomir-17. Scale bars represent 20 μm.
Targets of the members of the miR-17-92 cluster
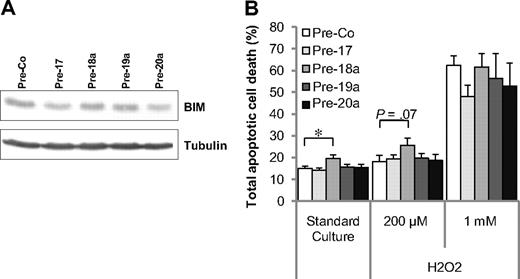
The miR-17-92 cluster was shown to target several proteins involved in cell cycle progression and apoptosis in hematopoietic and tumor cells. Among others, the miR-17-92 cluster was shown to target the protein Bim, a proapoptotic protein, which induces apoptosis of hematopoietic cells.25,26 Therefore, we first determined the effect of the individual members of the cluster on Bim expression in ECs. Overexpression of individual miR-17-92 cluster members caused only a minor reduction of Bim expression (Figure 4A), and most of the members of the miR-17-92 cluster did not exert an antiapoptotic effect on ECs under basal conditions or H2O2 stimulation (Figure 4B). Only miR-18a significantly enhanced apoptosis under basal conditions, but not after treatment with low and high concentrations of H2O2 (Figure 4B). Overall, these data indicate that Bim is not a functionally relevant target of the miR-17-92 cluster in ECs. Moreover, we observed a minor down-regulation of the antiangiogenic proteins CTGF and TSP-1 by overexpression of miR-18a and miR-19a but not by miR-17/20 in ECs (supplemental Figure 5).
Effect of members of the miR-17-92 cluster on apoptosis. (A) Expression of the proapoptotic protein Bim in HUVECs after transfection with precursors for the indicated miRs. A representative Western blot is shown; n = 3 experiments. (B) Detection of annexin-positive HUVECs after transfection with the miR precursors in the presence or absence of H2O2 for 14 hours; n = 3-11 experiments. *P < .05.
Effect of members of the miR-17-92 cluster on apoptosis. (A) Expression of the proapoptotic protein Bim in HUVECs after transfection with precursors for the indicated miRs. A representative Western blot is shown; n = 3 experiments. (B) Detection of annexin-positive HUVECs after transfection with the miR precursors in the presence or absence of H2O2 for 14 hours; n = 3-11 experiments. *P < .05.
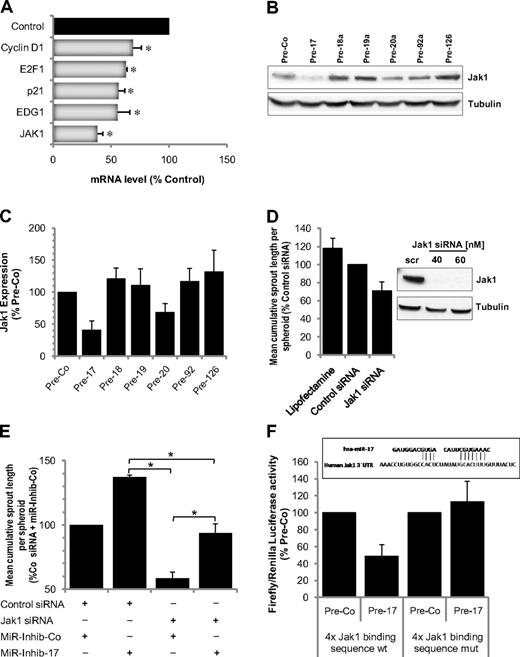
Having shown that miR-17 is the miR-17-92 member most significantly affecting angiogenesis in vitro and in vivo, we intended to identify relevant targets mediating the antiangiogenic activity of miR-17 in ECs. In a first approach, we performed a microarray mRNA profile. Overexpression of miR-17 significantly reduced several predicted targets, including the cell cycle inhibitor p21, the S1P receptor EDG1, and the protein kinase Janus kinase 1 (Jak1; Figure 5A; supplemental Tables 1, 2). The reduction of p21 mediated by miR-17 was associated with an increased proliferation of pre-miR-17 transfected ECs (supplemental Figure 6). In contrast, the reduction of EDG-1 expression did not affect the chemotactic response of the ECs to the EDG-1-ligand sphingosine-1-phosphate (supplemental Figure 7). Because Jak1 was efficiently down-regulated on mRNA as well as on protein level by miR-17 and the closely related miR-20a (Figure 5A-C) and we additionally demonstrated that inhibition of miR-17 increased Jak1 expression (data not shown), we further tested the function of Jak1 in ECs. siRNA-mediated silencing of Jak1 reduced angiogenesis in vitro (Figure 5D) and abrogated cytokine and growth factor-induced phosphorylation of Stat3 (supplemental Figure 8). Moreover, silencing of Jak1 partially reduced the proangiogenic effect mediated by miR-17 inhibitors (Figure 5E), indicating that Jak1 is one of the miR-17 downstream targets. To confirm a direct regulation of Jak1 by miR-17, we performed luciferase assays in which the miR-17 target sequence in the Jak1 3′-UTR was cloned in the luciferase 3′-UTR. Overexpression of miR-17 reduced luciferase activity but exhibited no effect on a mutated construct (Figure 5F), showing that miR-17 directly targets the Jak1 3′-UTR.
Identification of miR-17 targets. (A) mRNA expression of the putative targets after overexpression of Pre-17 in HUVECs for 24 hours; n = 3. *P ≤ .05. (B-C) Expression of Jak1 in HUVECs overexpressing the indicated miR precursors. (B) A representative Western blot. (C) Quantification; n = 4. (D) Effect of Jak1 silencing using 40nM siRNA on spheroid sprouting. An siRNA directed against firefly luciferase or cells treated with the transfection reagent was used as control; n = 5 or 6. Down-regulation of Jak1 protein 48 hours after siRNA transfection is shown in the representative Western blot. (E) Jak1 siRNA reduces the proangiogenic effect of miR-17 inhibition. HUVECs were transfected with Jak1 siRNA (40nM), miR-17 inhibitor (50nM), and the respective controls as indicated. Spheroids were generated and sprouting was quantified; n = 3. *P < .05. (F) Firefly luciferase activity normalized to Renilla luciferase activity measured in homogenates of HEK cells transfected with the wild-type (wt) or mutated luciferase constructs and Pre-17 or control Pre-miR (mutations were applied to underlined nucleotides). Measurements were done 48 hours after transfection; n = 4.
Identification of miR-17 targets. (A) mRNA expression of the putative targets after overexpression of Pre-17 in HUVECs for 24 hours; n = 3. *P ≤ .05. (B-C) Expression of Jak1 in HUVECs overexpressing the indicated miR precursors. (B) A representative Western blot. (C) Quantification; n = 4. (D) Effect of Jak1 silencing using 40nM siRNA on spheroid sprouting. An siRNA directed against firefly luciferase or cells treated with the transfection reagent was used as control; n = 5 or 6. Down-regulation of Jak1 protein 48 hours after siRNA transfection is shown in the representative Western blot. (E) Jak1 siRNA reduces the proangiogenic effect of miR-17 inhibition. HUVECs were transfected with Jak1 siRNA (40nM), miR-17 inhibitor (50nM), and the respective controls as indicated. Spheroids were generated and sprouting was quantified; n = 3. *P < .05. (F) Firefly luciferase activity normalized to Renilla luciferase activity measured in homogenates of HEK cells transfected with the wild-type (wt) or mutated luciferase constructs and Pre-17 or control Pre-miR (mutations were applied to underlined nucleotides). Measurements were done 48 hours after transfection; n = 4.
Discussion
The data of the present study demonstrate that all individual members of the miR-17-92 cluster tested block angiogenic sprouting of ECs in vitro. The findings that members of the miR-17-92 cluster inhibit angiogenesis and neovascularization are surprising and are in contrast to previous reports showing that overexpression of the miR-17-92 cluster promotes tumor angiogenesis.21 The proangiogenic activity was attributed to the block of the endogenous angiogenesis inhibitors TSP-1 and CTGF by miR-18 and miR-19 in tumor cells. The reduction of these inhibitors was suggested to tip the delicate balance of angiogenesis activators and inhibitors toward promotion of angiogenesis. Although we confirmed a slight regulation of TSP-1 and CTGF by overexpressing miR-18a and miR-19a in ECs, a proangiogenic activity of these miRs in ECs was not detected in any of the assays used. Thus, 3-dimensional spheroid sprouting was significantly inhibited by overexpression of both miR-18a and mir-19a and inhibition of miR-18a and miR-19a slightly increased sprouting in vitro and Matrigel plug neovascularization in vivo. One may speculate that targeting of other EC-intrinsic genes by miR-18a and miR-19a mediates these effects. In addition, one should note that TSP-1, which has been described to be the major mediator of the proangiogenic activity of miR-19 in tumor cells, can act in a context-dependent manner to either promote or block angiogenesis.27,28
Among the members of the miR-17-92 cluster tested in the present study, miR-17/20 exhibited the most profound effects in ECs in vitro and systemic blockade by antagomirs promoted neovascularization in vivo. Interestingly, the identical concentration of antagomir-17/20, which induced sprouting in implanted Matrigel plugs, did not enhance tumor angiogenesis in a LLC1 tumor model, suggesting that inhibition of miR-17/20 differentially affects neovascularization of Matrigel plugs compared with tumor angiogenesis. This observation might be explained by direct effects of miR-17/20 inhibition on tumor cells, thereby altering the secretome of tumor cells, which may antagonize the cell-intrinsic proangiogenic effects of the antagomirs on ECs. This hypothesis is supported by our findings that overexpression of miR-17 and miR-20a slightly enhanced the paracrine proangiogenic activity of tumor cells. Thus, a reduction of miR-17/20 expression in tumor cells might cause an antiangiogenic environment which counteracts the proangiogenic effects of miR-17/20 inhibition in ECs.
In addition, we cannot exclude a direct effect on tumor cell survival or proliferation mediated by the systemic effects of antagomir-17/20 injection, although antagomir-17/20 treatment of LLC1 cells in vitro had no profound effect on cell-cycle distribution (supplemental Figure 9). Other groups reported that a direct and repeated injection of antagomir-17 in the implanted tumors reduced tumor growth.20 However, in our study, the systemic intravenous injection of 1 or 2 doses of the rather low concentration of 8 mg/kg antagomir-17/20 did not reduce tumor growth, suggesting that these rather low systemically supplied concentrations of antagomir-17/20 are not sufficient to mediate such direct antiproliferative effects on tumors. One may envision that the slight increase in tumor weight and volume seen in our study might have been caused by an effect on the cell-cycle promoter cyclin D1, which has recently been shown to be targeted by miR-17 in tumor cells29 and might be increased under conditions of miR-17 inhibition.
In ECs, the combined overexpression of miR-17, miR-18a, and miR-20a was shown to rescue impaired EC functions induced by depletion of Dicer.7 However, in our experiments, none of the members of the miR-17-92 cluster exhibited a proangiogenic effect with respect to sprouting and particularly miR-17 suppressed the angiogenic activity of ECs in vitro and in vivo. Because our results are consistent with recent data in immortalized ECs showing that miR-17 blocks cell adhesion and migration,30 it is possible that the miR-17-92 cluster might have had specific effects under the stress imposed by the depletion of Dicer.
Because miR-17 exhibited the most profound effects on sprouting angiogenesis, we focused on the identification of the targets of this member of the miR-17-92 cluster. First, we confirmed previously identified targets of miR-17 and miR-20a and showed that the proapoptotic protein Bim and the cell-cycle inhibitor p21 were suppressed by overexpression of miR-17. However, apoptosis of ECs was only slightly reduced by miR-17 when using very high concentrations of hydrogen peroxide. The lack of a profound antiapoptotic effect of miR-17/20 mediated regulation of Bim is probably explained by the finding that Bim is not a major regulator of apoptosis in ECs but was shown to affect other cell types such as hematopoietic progenitor cells.31 In contrast to the modest effects on apoptosis, miR-17/20 significantly increased cell-cycle progression. Proliferation and sprouting are tightly regulated during vessel growth, as nicely illustrated in model organisms, such as the zebrafish embryo, where the formation of intersegmental vessels is associated with proliferation of stalk but not tip cells.
Moreover, cell-cycle regulators, such as p21, do not only function as cell-cycle inhibitors but also are required for migration of cells. Thus, a reduction of p21 as seen by overexpression of miR-17 may disturb the balance of coordinated vessel growth by activating proliferation but reducing migration. This may also explain why the effects of miR-17/20 are much more profound in spheroid sprouting models (Figure 1C), which mimic tip-stalk cell-mediated angiogenic growth, compared with the network forming activity detected by in vitro Matrigel assays (supplemental Figure 1A). However, the complex- and dose-dependent activity of p21 on cell cycle progression, apoptosis, and migration of ECs32 challenges to pinpoint p21 effects on vessel growth.
Screening for additional targets revealed several new targets of miR-17, namely, the tyrosine kinase Jak1, which was one of the most efficiently down-regulated targets in ECs. The Jak/Stat signaling pathways plays a crucial role in vascular homeostasis and disease.33 Jak1, Jak2, and Tyk2 are expressed in ECs34,35 and cardiomyocytes.36 In contrast, highest levels of Jak3 are found in the thymus.37 Unspecific inhibition of Jak/Stat signaling by AG-490 blocked angiogenesis.38 Moreover, mice with a cardiomyocyte-restricted STAT3 deletion showed a profoundly inhibited neovascularization capacity.39 The specific functions of Jak1 versus Jak2 are largely unknown, but selective disruption of the Jak1 gene demonstrates nonredundant roles of Jak1 in cytokine-responses of hematopoietic cells.40 In ECs, Jak1 siRNA was shown to reduce HIF protein expression.41 Our data further confirmed that Jak1 is indeed required for sprouting angiogenesis and growth factor-mediated activation of STAT3. The causal involvement of Jak1 was further evidenced by the findings that silencing Jak1 by siRNA partially prevented the proangiogenic activity of miR-17 inhibition. However, our data do not exclude a role of various additional targets, such as the EDG1 receptor, to the observed phenotype. Our findings that miR-17 mediated down-regulation of EDG-1 did not affect sphingosin-1-phosphate—induced migration is probably explained by compensation via the EDG-3 receptor that is highly expressed in ECs. However, EDG-1–deficient mice exhibit a profound defect in vascular development and die during embryonic development,42 suggesting that, under certain conditions, EDG-1 plays a unique role in the vasculature.
In conclusion, the findings of the present study provide novel insights into the complex regulation of angiogenesis and vascular growth by members of the miR-17-92 cluster and specifically miR-17/20. The differential effect of systemic inhibition of miR-17/20 on neovascularization of Matrigel plugs compared with LLC1 tumors suggests that the miR-17-92 cluster is differentially involved in the regulation of physiologic versus tumor angiogenesis. Conceptually, targeting specific members of the miR-17-92 family may provide an interesting therapeutic perspective to specifically enhance therapeutic angiogenesis.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Hellmut Augustin (DKFZ German Cancer Research Center, Heidelberg, Germany) for his advice regarding the tumor study and M. Muhly-Reinholz, T. Röxe, N. Konecny, A. Knau, and N. Reinfeld for expert technical assistance.
This work was supported by the European Research Council (Advanced grant “Angiomir”; S.D.) and the Excellence Cluster Cardiopulmonary System (Exc 147-1). C.D. is supported by a fellowship of the LOEWE Lipid Signaling program. C.U. and Y.R. are supported by the Transregio-Sonderforschungsbereich 23.
Authorship
Contribution: C.D., A.B., and A.F. performed research and analyzed data; A.S. and Y.R. provided support with the tumor model; C.U. and W.-K.H. provided support with the microarray; A.M.Z. and S.D. designed the study; and C.D., A.B., and S.D. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Stefanie Dimmeler, Institute of Cardiovascular Regeneration, Centre for Molecular Medicine, Goethe University of Frankfurt, Theodor-Stern-Kai 7, 60590 Frankfurt, Germany; e-mail: dimmeler@em.uni-frankfurt.de.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal