Abstract
Adaptive immune responses are characterized by substantial restructuring of secondary lymphoid organs. The molecular and cellular factors responsible for virus-induced lymphoid remodeling are not well known to date. Here we applied optical projection tomography, a mesoscopic imaging technique, for a global analysis of the entire 3-dimensional structure of mouse peripheral lymph nodes (PLNs), focusing on B-cell areas and high endothelial venule (HEV) networks. Structural homeostasis of PLNs was characterized by a strict correlation between total PLN volume, B-cell volume, B-cell follicle number, and HEV length. After infection with lymphocytic choriomeningitis virus, we observed a substantial, lymphotoxin (LT) β-receptor–dependent reorganization of the PLN microarchitecture, in which an initial B-cell influx was followed by 3-fold increases in PLN volume and HEV network length on day 8 after infection. Adoptive transfer experiments revealed that virus-induced PLN and HEV network remodeling required LTα1β2-expressing B cells, whereas the inhibition of vascular endothelial growth factor-A signaling pathways had no significant effect on PLN expansion. In summary, lymphocytic choriomeningitis virus-induced PLN growth depends on a vascular endothelial growth factor-A–independent, LT- and B cell–dependent morphogenic pathway, as revealed by an in-depth mesoscopic analysis of the global PLN structure.
Introduction
Peripheral lymph nodes (PLNs) are strategically positioned at the junctions of the blood and lymphatic vascular systems to efficiently contain and present microbes or derived peptides to passing lymphocytes for a rapid initiation of adaptive immune responses.1-4 The PLN parenchyma is filled with mobile lymphocytes organized in separate T- and B-cell microenvironments in the paracortex and follicles, respectively. PLNs contain antigen-presenting cells (APCs), in particular dendritic cells (DCs) in the T-cell area, which are embedded in a network of fibroblastic stromal cells (FRCs) and high endothelial venules (HEVs), the main port of entry for circulating lymphocytes.4-7
When live microbes or microbial products invade a host, draining PLNs undergo substantial morphologic changes to allow for increased recruitment of naive cells. Macroscopically, this is manifested in PLN swelling and an increase in the lymph and blood vasculature.8-15 In parallel, immunohistologic analysis of the distribution of APC, lymphocyte subsets, and stromal markers points to a substantial microenvironmental reorganization in draining PLNs.12,16 These changes are in part caused by a temporary shutdown of lymphocyte egress, recruitment of new lymphocytes and activated DCs, as well as retention and clonal expansion of antigen-specific lymphocytes.4,17 Expression levels of homeostatic chemokines, such as CCL19 and CCL21, decrease in inflamed lymphoid tissue, either through interferon-γ–dependent mechanisms or through destruction of the stromal network producing these chemotactic factors. As an example, infection with the model pathogen lymphocytic choriomeningitis virus (LCMV) results in 10-fold–reduced CCL19 and CCL21 mRNA levels, precipitating the loss of lymphoid organization.17,18
The inflammation-induced PLN expansion is an almost-unparalleled process of tissue remodeling regarding its rapidity and the extent of size increase in adult organisms. The increase in blood vasculature implies a role for the proangiogenic factor vascular endothelial growth factor A (VEGF-A) during PLN expansion. Accordingly, in delayed-type hypersensitivity responses or adjuvans-mediated PLN remodeling, VEGF-A regulates lymph and blood vessel angiogenesis in B cell–dependent and –independent manners.12,14,15 Activated DCs also contribute to HEV expansion in a VEGF-A–dependent manner.14 FRCs contribute to PLN growth through secretion of VEGF-A after stimulation of their lymphotoxin β receptor (LTβR).19 LTβR is furthermore expressed by HEV and lymphatic vessels and is involved in regulation of the HEV phenotype in steady state and inflammation.13,20
The precise contribution of these morphogenic cells and factors to PLN growth is likely to vary according to the inflammatory stimuli used. For example, because LCMV infection leads to a down-regulation of FRC and DC numbers in draining PLNs,17 these cells are unlikely to decisively contribute to global lymphoid tissue reorganization in this viral model. It is furthermore unknown whether B cells, or other blood-borne cells, are involved in LCMV-induced tissue remodeling, as observed in noninfectious models.12 Thus, the roles for VEGF-A and the LTβR ligand LTα1β2 on B cells, or other B-cell–derived factors, during lymphoid remodeling in viral infections have not been analyzed fully to date.
A thorough analysis of tissue restructuring during inflammation relies on a precise quantification of lymphoid architecture. Traditionally, the spatial and quantitative relationship between vascular structures and lymphoid compartments has been quantified by use of 2-dimensional tissue sectioning.12-16,21 A limitation of this approach is the asymmetric distribution of vascular structures and T- and B-cell areas in PLN cortex and medulla, which results in high variability of observable structures in 2-dimensional sections according to the dissection plane. Furthermore, only a limited number of tissue sections is typically analyzed, rendering global statements on lymphoid restructuring less precise. A complete 3-dimensional (3D) overview of the internal PLN structure, including lymphocyte subcompartments and vascular networks, is therefore required to analyze with high precision the molecular mechanisms underlying homeostatic regulation and inflammation-induced changes. Mesoscopic imaging is ideally suited to probe objects of 0.5 to 15 mm in diameter, which are too large for conventional microscopic imaging, that is, confocal or 2-photon microscopy (2PM). A number of mesoscopic imaging techniques, including selective plane illumination microscopy, ultramicroscopy, and optical projection tomography (OPT), have been developed in recent years and successfully applied to embryos and adult organs of small vertebrates.22-27
We hypothesized that mesoscopic imaging could provide a comprehensive quantification of the entire PLN structure in homeostasis and inflammation induced by the model pathogen LCMV. Here, we have applied OPT to whole-mount fluorescently labeled murine PLN to obtain quantitative structural 3D information on PLN. We provide evidence that growth of the PLN and HEV network during LCMV infection depends on LTα1β2 signals provided to a large extent by B cells, whereas blocking VEGF-A signaling does not have a significant effect on tissue remodeling.
Methods
Mice
We purchased 6- to 8-week-old C57BL/6 and FVB mice from Charles River Laboratories and Harlan Laboratories. B cell–deficient JHT mice28 and LTβ−/− mice29 were obtained from the Institut für Labortierkunde, University of Zurich. DOCK2−/− mice30 were bred at the specific pathogen-free mouse facility at the animal facility of the Department of Clinical Research, University of Bern. Experiments with LCMV infection were carried out in the animal facility of the Institute for Immunobiology, Kantonal Hospital. All animal experiments were carried out in accordance with federal and Kantonal legislation on animal protection and were approved by the institutional review board of the University of Bern.
Antibodies
For a complete listing of monoclonal antibodies (mAbs) used in this study, refer to supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Viral infection
The LCMV WE strain, which was obtained from Rolf M. Zinkernagel (University of Zürich), was propagated on L929 cells at a low multiplicity of infection and quantified as previously described.31 Mice were infected with 200 plaque-forming units (pfu) of LCMV WE strain intravenously and analyzed at the indicated time points after infection. For infection with vesicular stomatitis virus (Indiana strain, provided by Rolf M. Zinkernagel), 2 × 106 pfu were injected intravenously as described.17
Quantitative polymerase chain reaction
Quantitative polymerase chain reaction (qPCR) of cDNA isolated from control and LCMV-infected PLNs was carried out with mouse VEGF-A and VEGFR2 primers by the use of LightCycler FastStart DNA master SYBR Green I kit (Roche Diagnostics) on a LightCycler 1.5 (Roche Diagnostics) as described.17 The primer sequences for VEGF-A and VEGR2, were, respectively, 5′ primer CAGGCTGCTGTAACGATGAA and 3′ primer TTTCTTGCGCTTTCGTTTTT and 5′ primer GGCGGTGGTGACAGTATCTT and 3′ primer GTCACTGACAGAGGCGATGA.
In vivo antibody and inhibitor treatment
For the depletion of B cells, C57BL/6 mice received intraperitoneal injections of anti-CD20 antibody or isotype control antibody (250 μg/mouse; provided by Biogen Idec) on day −10 before infection. For blocking of VEGF signaling, we injected neutralizing anti–mouse VEGF-A mAb at a dose of 2 × 100 μg/mouse on day 0 and 3 after infection, from ReliaTech (clone MAB0705) or BioLegend (2G11). Similar doses were previously used to block VEGF-A in mice.15 Anti-VEGFR2 (clone DC101; BioXcell) was injected on day 0 and 3 after infection (2 × 500 μg/mouse). For blockade of LTβR signaling, naive mice or LCMV-infected mice received intraperitoneal injections of soluble LTβR-immunoglobulin (Ig) or control Ig (100 μg/mouse; provided by Biogen Idec) on day 0 and day 3 after infection. The VEGFR/PDGFR inhibitor sunitinib (Selleck) was used at 35 mg/kg in citrate-buffered water and administered daily by oral gavage for 7 days.
A contact hypersensitivity response toward oxazolone was induced in the ear skin of 8-week-old female FVB mice as described.32 For more details, refer to our supplemental Methods.
Adoptive B-cell transfers
Single-cell suspensions from spleens of LTβ−/− mice and C57BL/6 control mice were subjected to magnetic cell selection with the use of anti-B220 MACS-beads (Miltenyi Biotec). Purity of the cell preparations was analyzed by flow cytometry. B-cell preparations (> 95% B220+) were washed twice with ice-cold BSS and resuspended in BSS at a concentration of 4 × 107 cells/mL. JHT recipient mice were injected intravenously with 1.5 to 2 × 107 cells in 500 μL of BSS on day 2 before and the day of infection with LCMV.
Whole-mount staining for secondary lymphoid organs and preparation for OPT
Fifteen minutes before they were killed, control and LCMV-infected mice received an intravenous injection of fluorescently labeled MECA-79 or MECA-89 (12-15 μg) to label the HEV network. Afterward, PLN, mesenteric lymph nodes (MLN), and Peyer patches (PP) were carefully excised and surrounding tissue was removed with a stereomicroscope. Cleaned organs were fixed overnight in 0.4% paraformaldehyde (Electron Microscopy Sciences) in phosphate-buffered saline (PBS) under slow shaking at 4°C. After 3 washing steps in washing buffer (PBS/0.1% Triton X100/0.05% sodium azide) for 1 hour each, PLNs were incubated with 0.0025% collagenase for 30 minutes at 37°C. Collagenase activity was stopped by addition of cold fetal calf serum, followed by 3 washes with cold PBS for 1 hour each. PLNs were then incubated with AlexaFluor 488–conjugated anti-B220 (0.67 μg/mL) in washing buffer for 10 days at 4°C with gentle rotation. Stained PLN were washed in PBS, mounted in 1.5% ultrapure low-melting agarose (Invitrogen), and transferred to methanol for 4 to 6 hours before they were transferred to BABB (ie, benzyl alcohol and benzyl benzoate in a 1:2 ratio) for at least 24 hours. This step “clears” the tissue by strongly reducing light scattering and absorption.25 For verification of whole-mount staining procedure efficiency, whole-mount–stained PLNs were frozen as discussed previously, and 8-μm cryostat sections were directly observed under the fluorescence microscope after colabeling with MECA-79 or anti-IgM mAbs.
OPT scanning and software-based 3D reconstruction and quantification
OPT scanning was performed on BABB-immersed PLN according to the manufacturer's instructions (Bioptonics) with use of the following filter sets: exciter 425/40, emitter LP475 for autofluorescent signal; exciter 480/20, emitter LP515 for green fluorescent signal; and exciter 545/30, emitter 617/75 for red fluorescent signal. Raw data were reconstructed into 3D voxel datasets by use of the manufacturer's software (NRecon). Reconstructed virtual xyz data sets were exported as .TIFF or .bmp files and analyzed with IMARIS (Bitplane) for isosurface rendering of total PLN volume and B-cell follicles. IMARIS reconstructions were carefully adjusted to fit original NRecon reconstructions. B-cell follicles were visually inspected in the 3D reconstructions and considered an individual follicle on the basis of the near-spherical shape in xyz dimensions. To differentiate between follicles with “connecting” channels and superlarge, fused follicles, particular weight was given on the intranodal expansion of the follicle. The total HEV length and thickness, branching points, and individual/average segment lengths were analyzed by use of the Filament tracer module (Bitplane).
Statistical analysis
Student t test, 1-way analysis of variance (ANOVA), and linear regression were used to determine statistically significant differences (GraphPad Software). P values of less than .05 were considered statistically significant.
Results
Mesoscopic analysis of homeostatic lymphoid tissue
To carry out OPT-based mesoscopic imaging of internal PLN structures, we developed suitable whole-mount staining protocols (Figure 1A; supplemental Figure 1). From labeled PLNs, we obtained virtual reconstructions, which were analyzed by the use of software programs commonly used in confocal image analysis. From these data, we were able to obtain quantifiable information on total PLN volume, B-cell follicle number and shape, and total HEV vasculature (Figure 1B).
Mesoscopic examination of naive PLN volume, B-cell follicle structure, and HEV network. (A) Workflow of OPT analysis of PLN architecture. PLN labeled with B220-Alexa488 and MECA-79-Alexa594 mAbs were dehydrated, cleared in BABB, and imaged in an OPT scanner as described in “Whole-mount staining for secondary lymphoid organs and preparation for OPT” (top). In some experiments, MECA-79-Alexa568 was used with similar results. After OPT image acquisition, samples can be further analyzed by high-resolution laser scanning microscopy (LSM) or rehydrated for flow cytometry analysis (bottom). As seen on the left (OPT), the current resolution clearly identifies HEV but not individual endothelial cells as seen when high-resolution LSM is used. Scale bar in LSM = 40 μm. (B) Schematic representation of primary OPT images (left; shown is 1 of 400 raw images before 3-dimensional [3D] reconstruction), 3D reconstruction (middle), and quantification of fluorescently labeled structures (right). Before OPT imaging, PLNs were whole-mount labeled with B220-Alexa488 (for B-cell follicles) and MECA-79-Alexa594 (anti-PNAd for HEV) mAbs and processed as described in “OPT scanning and software-based 3D reconstruction and quantification.” The length of the long axis of the inguinal PLN shown is 2.4 mm.
Mesoscopic examination of naive PLN volume, B-cell follicle structure, and HEV network. (A) Workflow of OPT analysis of PLN architecture. PLN labeled with B220-Alexa488 and MECA-79-Alexa594 mAbs were dehydrated, cleared in BABB, and imaged in an OPT scanner as described in “Whole-mount staining for secondary lymphoid organs and preparation for OPT” (top). In some experiments, MECA-79-Alexa568 was used with similar results. After OPT image acquisition, samples can be further analyzed by high-resolution laser scanning microscopy (LSM) or rehydrated for flow cytometry analysis (bottom). As seen on the left (OPT), the current resolution clearly identifies HEV but not individual endothelial cells as seen when high-resolution LSM is used. Scale bar in LSM = 40 μm. (B) Schematic representation of primary OPT images (left; shown is 1 of 400 raw images before 3-dimensional [3D] reconstruction), 3D reconstruction (middle), and quantification of fluorescently labeled structures (right). Before OPT imaging, PLNs were whole-mount labeled with B220-Alexa488 (for B-cell follicles) and MECA-79-Alexa594 (anti-PNAd for HEV) mAbs and processed as described in “OPT scanning and software-based 3D reconstruction and quantification.” The length of the long axis of the inguinal PLN shown is 2.4 mm.
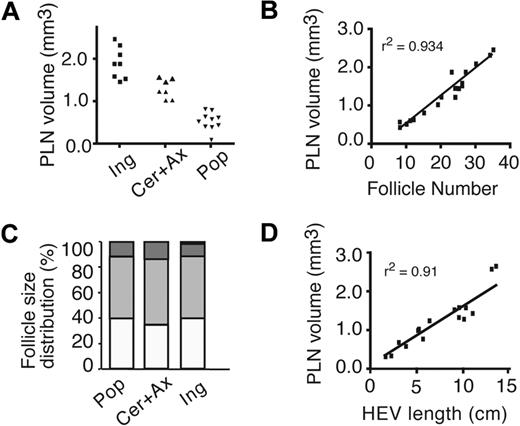
On the basis of total volume, mouse PLNs were classified as small (popliteal LN), medium (cervical and axillary LN), and large (inguinal LN; Figure 2A). B-cell follicles were found polarized in the PLN cortex, with numbers of individual B-cell follicles per PLN ranging from less than 10 to more than 30 (Figure 2B; supplemental Video 1). Although 25% to 60% of B-cell follicles per PLN were clearly separated from another, 40% to 70% of follicles appeared linked via narrow “connecting channels,” joining up to 6 individual follicles. A third type of follicle (5%-20%) was atypically shaped with no clearly identifiable spherical outline and appeared to have arisen from fusion of adjacent follicles (supplemental Figure 2A). The ratio between the number of B-cell follicles per PLN and the total PLN volume remained remarkably constant, indicating that larger PLNs contain a greater number of, rather than larger, B-cell follicles (Figure 2B).
Homeostatic PLN structure. (A) Classification of PLN into small (Pop: popliteal), medium (Cer + Ax: cervical and axillary), and large (Ing: inguinal). Each symbol represents 1 PLN. (B) Correlation between PLN volume and number of B-cell follicles. Each square represents 1 PLN. (C) Normalized size distribution of individual B-cell follicles. ▭ indicate 1 to 5 × 106 μm3;  , 5 to 15 × 106 μm3;
, 5 to 15 × 106 μm3;  , 15 to 25 × 106 μm3; and ▬, greater than 25 × 106 μm3. (D) Correlation between PLN volume and total HEV length. Each square represents 1 PLN.
, 15 to 25 × 106 μm3; and ▬, greater than 25 × 106 μm3. (D) Correlation between PLN volume and total HEV length. Each square represents 1 PLN.
Homeostatic PLN structure. (A) Classification of PLN into small (Pop: popliteal), medium (Cer + Ax: cervical and axillary), and large (Ing: inguinal). Each symbol represents 1 PLN. (B) Correlation between PLN volume and number of B-cell follicles. Each square represents 1 PLN. (C) Normalized size distribution of individual B-cell follicles. ▭ indicate 1 to 5 × 106 μm3;  , 5 to 15 × 106 μm3;
, 5 to 15 × 106 μm3;  , 15 to 25 × 106 μm3; and ▬, greater than 25 × 106 μm3. (D) Correlation between PLN volume and total HEV length. Each square represents 1 PLN.
, 15 to 25 × 106 μm3; and ▬, greater than 25 × 106 μm3. (D) Correlation between PLN volume and total HEV length. Each square represents 1 PLN.
This finding was confirmed when we analyzed the size distribution of B-cell follicles in small, medium, and large PLNs. Irrespective of total PLN volume, approximately 50% of individual B-cell follicles were between 5 and 15 × 106 μm3, with the remaining 50% between 1 and 5 and 15 and 25 × 106 μm3, respectively (Figure 2C). A similar consistent ratio also was maintained between total PLN volume and total volume of B cells (ie, the sum of all B-cell follicle volumes per PLN), with B-cell follicles typically occupying 10% of total PLN volume (supplemental Figure 2B-C). Assuming a comparable B-cell density in different B-cell follicles, a medium-sized B-cell follicle of 10 × 106 μm3 (∼ 225 × 225 × 200 μm) contains approximately 7 × 104 B cells.
In 3D reconstructions of PLNs, we also could analyze the dense HEV network, which occupied most of the paracortex, albeit without overlapping with B-cell follicles, and continued in close proximity to the medullar region (supplemental Video 1). As with B-cell follicle numbers, we observed a constant ratio between total PLN volume and HEV length (Figure 2D). The combined length of the HEV network in a single inguinal LN was typically around 10 cm, underlining the extensive network structure and its function as continuous port of entry for large numbers of lymphocytes. The total number of branching points of HEV per PLN was proportional to the total HEV length (V.K. and J.V.S., unpublished data, 2009), indicative of comparable network structure throughout all PLN sizes.
We next investigated the requirements for the proper formation of HEV and B-cell follicles. The HEV network was comparable in length in absence of B cells or when T cells were less abundant (in JHT, CCL19, and CCL21-deficient plt/plt and CCR7−/− PLNs; unpublished data). Formation of spherical-shaped follicles required active migration of B cells because PLNs isolated from mice lacking the promigratory Rac guanine exchange factor DOCK230 lacked discernible B-cell follicles (supplemental Figure 3A; supplemental Video 2). Mesoscopic analysis of PP and MLNs revealed an overall organization comparable with PLNs, with polarized B-cell follicles and dense HEV networks occupying the interfollicular space (supplemental Figure 3B; supplemental Videos 3-4). MLNs contain both PNAd+ and MAdCAM-1+ HEV, each subset constituting approximately one-half of the total vascular network length (supplemental Figure 3C). Although most PNAd+ vessels were located in the cortical area, MAdCAM-1+ HEV occupied the medullar and lateral regions of MLNs (supplemental Video 5). A subset of HEV (10%-12%) was found to express both vascular addressins and localized to the cortical region, presumably to facilitate entry of blood-borne B cells into adjacent follicles.
In summary, OPT-based mesoscopic imaging provides a comprehensive overview of the internal organization of PLNs and other secondary lymphoid organs (SLOs). Our data support a model in which the individual B-cell follicle sizes are controlled by homeostatic mechanisms independent of total PLN size. Furthermore, we observed a gradual increase in average B-cell follicle size in MLN and PP, which reflects the increased abundance of B cells in these organs.
PLN remodeling during viral infection
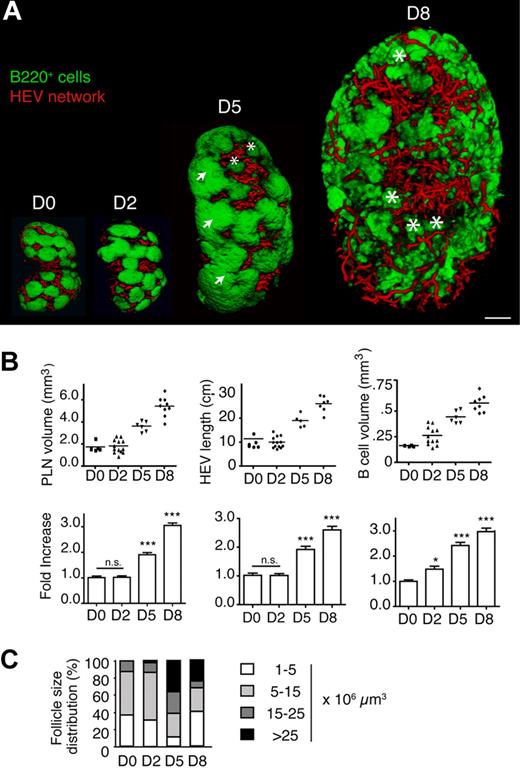
LCMV is a noncytopathic virus with strong tropism for SLOs, such as spleen and PLNs, where it causes a gradual loss of T- and B-cell zone integrity with maximal disruption between days 8 and 12 after infection.17,18 To evaluate the kinetics of LCMV-specific morphologic changes in the B-cell compartment and HEVs of PLNs, mice were infected intravenously with 200 pfu LCMV. On day 0, 2, 5, 8, 12, and 24 after infection, we isolated inguinal, popliteal, and axillary PLNs from infected mice after labeling HEV and B cells as described previously. OPT reconstructions showed remarkable remodeling of PLN size and internal structure during the course of infection. In all subsequent figures, inguinal PLNs are depicted, although comparable results were obtained with popliteal and axillary PLNs (supplemental Figure 5; V.K. and J.V.S., unpublished data, 2009). After a delay of 2 days, LCMV-infected PLNs started to expand 3-fold in size until day 8 after infection before contracting until day 24 after infection (Figure 3A; supplemental Figure 4). B-cell follicles became enlarged over day 2 and 5 after infection before breaking up into small clusters on day 8 after infection (Figure 3A-B; supplemental Videos 6-8), paralleled by an increase in absolute B-cell numbers.17 As a result, the ratio of total B-cell volume to PLN volume transiently increased on day 2 after infection, where B cells occupied 15% instead of 10% of the total PLN volume. “Superlarge” follicles with volumes of more than 25 × 106 μm3 appeared from day 2 after infection onwards, which most likely formed through fusion of several follicles (Figure 3A,C). The percentage of large and superlarge follicles increased on day 5 after infection, with a concomitant decrease in the smaller- and medium-sized follicles (Figure 3C). In contrast, on day 8 after infection, most B-cell accumulations lost the typical spherical shape of B-cell follicles and were smaller than on day 0, with some remaining superlarge B-cell follicles. Between days 5 and 8 after infection, these B-cell clusters lost their polarized localization and became distributed throughout the HEV-rich paracortex (Figure 3A; supplemental Videos 7-8). Thus, LCMV infection of PLNs was accompanied by loss of polarized medium-sized B-cell follicles, which likely reflects decreased CXCL13 expression at the peak of an LCMV infection.17,18
Structural changes in inguinal PLN volume, HEV network, and B-cell follicle size and distribution during LCMV infection. (A) Representative OPT images of B220+ areas (green) and HEVs (red) showing an initial increase in the B-cell follicle size on day (D2) after infection followed by incremental loss of regular follicle organization by day 8 after infection (D8). Arrows indicate superlarge follicles (> 25 × 106 μm3); asterisks, small B-cell clusters (< 5 × 106 μm3). Scale bar = 400 μm. (B top) Absolute volume, B-cell volume, and HEV length of inguinal PLN during LCMV infection. Each dot represents 1 PLN. (Bottom) Fold-increase compared with day 0. Data are shown as mean ± SEM *P < .05, ***P < .001, n.s., not significant (1-way ANOVA). (C) Change in B-cell follicle size distribution during LCMV infection. B-cell follicle sizes were classified as in Figure 2C. Pooled from a total of 6 to 12 inguinal, popliteal, and axillary PLNs from 3 to 7 mice for each time point.
Structural changes in inguinal PLN volume, HEV network, and B-cell follicle size and distribution during LCMV infection. (A) Representative OPT images of B220+ areas (green) and HEVs (red) showing an initial increase in the B-cell follicle size on day (D2) after infection followed by incremental loss of regular follicle organization by day 8 after infection (D8). Arrows indicate superlarge follicles (> 25 × 106 μm3); asterisks, small B-cell clusters (< 5 × 106 μm3). Scale bar = 400 μm. (B top) Absolute volume, B-cell volume, and HEV length of inguinal PLN during LCMV infection. Each dot represents 1 PLN. (Bottom) Fold-increase compared with day 0. Data are shown as mean ± SEM *P < .05, ***P < .001, n.s., not significant (1-way ANOVA). (C) Change in B-cell follicle size distribution during LCMV infection. B-cell follicle sizes were classified as in Figure 2C. Pooled from a total of 6 to 12 inguinal, popliteal, and axillary PLNs from 3 to 7 mice for each time point.
In contrast to the B-cell volume, the total inguinal PLN volume and HEV length were not increased on day 2 after infection (Figure 3B). On day 5 and 8 after infection, PLN volume, B-cell volume and total MECA-79+ HEV length steadily augmented until reaching a remarkably constant 3-fold increase in all 3 parameters (Figure 3B). The increased HEV network also supported increased recruitment of naive lymphocytes (V.K. and J.V.S., unpublished data, 2009). We did not observe appearance of MAdCAM-1+ vessels in control or LCMV-infected PLNs (V.K. and J.V.S., unpublished data, 2009).
B cells mediate PLN and HEV network expansion during LCMV infection
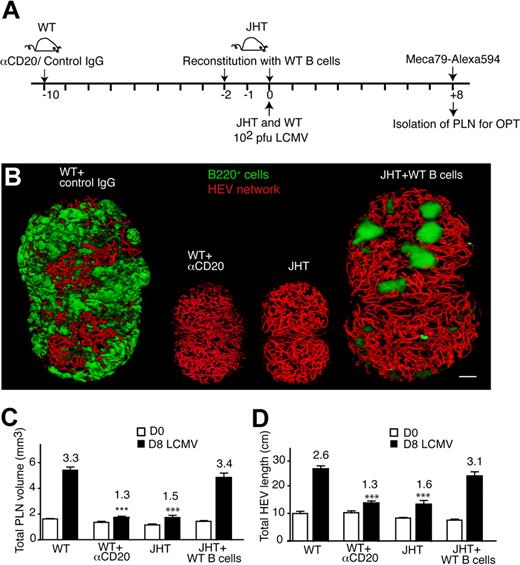
The early B-cell increase during LCMV infection prompted us to investigate the involvement of these cells in the subsequent enlargement of draining PLN. Because B cells do not contribute efficiently to the initial clearance of the virus from SLOs after low-dose infection,33,34 B cell–deficient JHT mice were used to determine the role of B cells for LCMV-mediated PLN and HEV network expansion (Figure 4A). PLN expansion during LCMV infection was markedly diminished in the absence of B cells in comparison with control PLN on day 8 after infection (Figure 4B-C). Similarly, the increase in total HEV length also was reduced compared with wild-type (WT) PLN, albeit not completely abolished (Figure 4D). Comparable results were obtained when we depleted B cells in WT mice using anti–mouse-CD20 mAb and performed an OPT analysis of LCMV-infected PLN on day 0 and 8 after infection as described previously (Figure 4A). Similar to JHT mice, the growth of B cell–depleted PLN and the HEV network on day 8 after infection was clearly lower than in control PLN (Figure 4B-D).
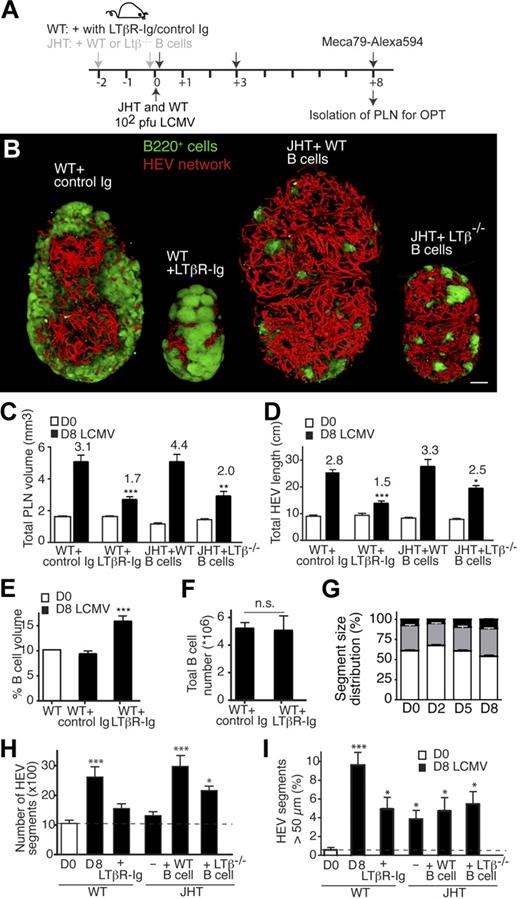
B-cell requirement for LCMV-triggered PLN expansion. (A) Experimental layout for B-cell depletion in WT mice (αCD20 mAb; 250 μg/mouse on day −10) versus B-cell reconstitution and LCMV infection in JHT mice. A total of 2 × 15 to 20 × 106 B cells were injected per JHT recipient on day −2 and 0. (B) Representative OPT images of day 8 (D8)–LCMV-infected inguinal PLNs of WT, WT + αCD20, JHT, and JHT + WT B cells. Scale bar = 400 μm. (C) Inguinal LN volume on day 0 (D0, □) and day 8 (D8, ■) after infection of WT mice, or WT mice + αCD20 mAb or JHT mice reconstituted with WT B cells on day −2 and day 0 after infection. ***P < .001 compared with day 8 LCMV WT (1-way ANOVA). (D) Total HEV network length on day 0 and day 8 after infection as in panel A. All day 8 (D8) values in panels A and B were significantly greater than on corresponding day 0 (Student t test). ***P < .001 compared with day 8 LCMV WT (1-way ANOVA). Numbers indicate fold increase over day 0. No significant difference was found in panels A and B between day 8 “WT” and “JHT + WT B cells” nor between “JHT” and “WT + αCD20” (1-way ANOVA). Pooled from 3 to 8 PLNs from 2 to 6 mice in 2 independent experiments. Data are shown as mean ± SEM.
B-cell requirement for LCMV-triggered PLN expansion. (A) Experimental layout for B-cell depletion in WT mice (αCD20 mAb; 250 μg/mouse on day −10) versus B-cell reconstitution and LCMV infection in JHT mice. A total of 2 × 15 to 20 × 106 B cells were injected per JHT recipient on day −2 and 0. (B) Representative OPT images of day 8 (D8)–LCMV-infected inguinal PLNs of WT, WT + αCD20, JHT, and JHT + WT B cells. Scale bar = 400 μm. (C) Inguinal LN volume on day 0 (D0, □) and day 8 (D8, ■) after infection of WT mice, or WT mice + αCD20 mAb or JHT mice reconstituted with WT B cells on day −2 and day 0 after infection. ***P < .001 compared with day 8 LCMV WT (1-way ANOVA). (D) Total HEV network length on day 0 and day 8 after infection as in panel A. All day 8 (D8) values in panels A and B were significantly greater than on corresponding day 0 (Student t test). ***P < .001 compared with day 8 LCMV WT (1-way ANOVA). Numbers indicate fold increase over day 0. No significant difference was found in panels A and B between day 8 “WT” and “JHT + WT B cells” nor between “JHT” and “WT + αCD20” (1-way ANOVA). Pooled from 3 to 8 PLNs from 2 to 6 mice in 2 independent experiments. Data are shown as mean ± SEM.
To further explore the function of B cells, we carried out reconstitution experiments in which 1.5 to 2 × 107 WT B cells were adoptively transferred into JHT mice on day −2 and 0 of LCMV infection (Figure 4A). We then performed an OPT analysis of draining PLNs on days 0 and 8 after infection as described previously. Flow cytometry and immunohistologic analysis in reconstituted PLN on day 8 after infection showed that transferred B cells were unevenly distributed and represented less than 5% of total lymphocytes in JHT PLN, compared with greater than 50% in WT PLN (Figure 4B; supplemental Figure 6). Despite the low B-cell number and their altered distribution, the adoptively transferred B cells were sufficient to completely restore the increase in total PLN volume, HEV length on day 8 after infection to levels of WT PLN, although the cellular composition shifted to increased T-cell numbers (Figure 4B; supplemental Figure 6). When only 4 × 106 B cells were adoptively transferred into JHT mice, the LCMV-induced PLN size and HEV network increase was reduced but still 1.25- to 1.5-fold greater compared with LCMV-infected JHT PLNs in the absence of B cells (unpublished data). Taken together, although the lack of B cells does not affect the HEV network in noninflamed PLNs, our data point out a central role for a threshold level of B cells to stimulate full PLN and HEV network growth during LCMV infection. Our data furthermore show that even very low numbers of B cells have a detectable effect on PLN remodeling and that B cell–independent mechanisms leading to PLN remodeling exist.
Blocking VEGF-A signaling does not inhibit LCMV-induced PLN remodeling
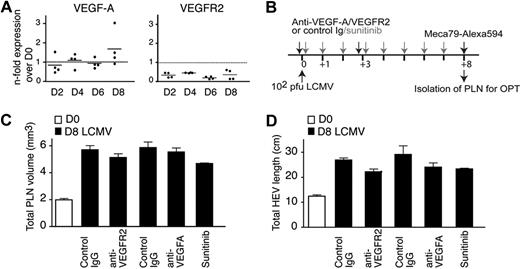
Because activated B cells have been previously shown to secrete VEGF-A and induce PLN remodeling,12 we assessed the role for VEGF-A for PLN and HEV network growth. Analysis by qPCR from LCMV-infected PLNs did not uncover increased expression of VEGF-A or its main receptor VEGFR-2 (Figure 5A), whereas we readily detected increased VEGF-C and LYVE-1 expression during LCMV infection, in line with increased lymphatic endothelium growth (V.K., R.D., J.V.S., unpublished data). We next performed LCMV infections in the presence of blocking Abs against VEGFR-2 or VEGF-A or in presence of the VEGFR/PDGFR inhibitor sunitinib (Figure 5B).35 In neither case did we observe a significant inhibition of PLN size increase and HEV network expansion on day 8 after infection (Figure 5C-D), whereas sunitinib readily decreased ear swelling in a delayed-type hypersensitivity model (supplemental Figure 7). Taken together, under the conditions used here, we could not find evidence for a central role of VEGF-A during LCMV-induced PLN expansion.
VEGF-A expression and inhibition during LCMV-induced PLN remodeling. (A) qPCR analysis of VEGF-A and VEGFR2 expression in LCMV-infected PLNs. Two PLNs from 2 mice per time point were analyzed in duplicates. (B) Experimental layout for VEGF signaling blocking and LCMV infection in WT mice. Mice received 2 injections on day 0 and 3 after infection of Abs as described in “In vivo antibody and inhibitor treatment” or daily gavage of the VEGFR/PDGFR-inhibitor sunitinib. (C) Inguinal LN volume on day 0 (D0) or day 8 (D8) after infection after treatment of mice with control Ig or anti-VEGFR2, anti–VEGF-A Abs, or sunitinib. Examples were pooled from 1 to 2 independent experiments with 3 to 5 mice per treatment. (D) Total HEV length on day 0 (D0) or day 8 (D8) after infection as in panel C. No significant difference was found in panels C and D between day 8 control Ig and Ab- or inhibitor-treated values (1-way ANOVA).
VEGF-A expression and inhibition during LCMV-induced PLN remodeling. (A) qPCR analysis of VEGF-A and VEGFR2 expression in LCMV-infected PLNs. Two PLNs from 2 mice per time point were analyzed in duplicates. (B) Experimental layout for VEGF signaling blocking and LCMV infection in WT mice. Mice received 2 injections on day 0 and 3 after infection of Abs as described in “In vivo antibody and inhibitor treatment” or daily gavage of the VEGFR/PDGFR-inhibitor sunitinib. (C) Inguinal LN volume on day 0 (D0) or day 8 (D8) after infection after treatment of mice with control Ig or anti-VEGFR2, anti–VEGF-A Abs, or sunitinib. Examples were pooled from 1 to 2 independent experiments with 3 to 5 mice per treatment. (D) Total HEV length on day 0 (D0) or day 8 (D8) after infection as in panel C. No significant difference was found in panels C and D between day 8 control Ig and Ab- or inhibitor-treated values (1-way ANOVA).
LTβ on B cells regulates LCMV-induced PLN remodeling
LT is an important morphogenic factor for PLN homeostasis and inflammation-induced hypercellularity.20 To address the role of LT for PLN remodeling during LCMV infections, WT mice received LTβR-Ig, a general inhibitor of LTβR signaling, on days 0 and 3 after LCMV infection (Figure 6A). Blocking LTβR signaling caused a significant reduction in LCMV-induced PLN size and HEV length increase compared with mice treated with control Ig (Figure 6B-D). The 3D reconstructions of LCMV-infected PLN in LTβR-Ig–treated mice showed an increase in B-cell follicle sizes on day 8 after infection, resulting in massive fusion of these follicles and appearance of super-large follicles (supplemental Video 9). The absolute B-cell volume on day 8 after infection was similar to the one observed in mice who had received control Ig (V.K. and J.V.S., unpublished data, 2009), resulting in a greater percentage of B-cell volume to PLN volume (Figure 6E). This was corrobated by flow cytometry–based analysis of absolute B-cell numbers from LTβR-Ig–treated PLN compared with the control Ig-treated PLN (Figure 6F). These observations suggest that LTβR-Ig treatment does not disrupt initial B-cell accumulation in LCMV-infected PLN but impairs subsequent PLN and HEV network growth.
LT signaling during LCMV-induced PLN remodeling. (A) Experimental layout for LT blocking in WT mice and B-cell reconstitution in JHT mice, followed by LCMV infection. WT mice received 2 injections on day 0 and 3 after infection of 100 μg of control or LTβR-Ig. JHT mice were reconstituted with WT or LTβ−/− B cells as described in Figure 4A. (B) Representative OPT images of inguinal LN isolated from day 8 (D8) LCMV-infected WT, WT + LTβR-Ig, JHT + WT B cells, and JHT + LTβ−/− B cells mice. Scale bar = 400 μm. (C) Inguinal LN volume on day 0 (D0) or day 8 (D8) after infection after treatment of mice with control Ig or LTβR-Ig or in JHT mice reconstituted with WT or LTβ−/− B cells. (D) Total HEV length on day 0 (D0) or day 8 (D8) after infection as in panel C. All day 8 values in panels C and D were significantly greater than on corresponding day 0 (Student t test). *P < .05, **P < .01, ***P < .001 comparing day 8 LCMV “WT + control Ig” vs “WT + LTβR-Ig” and “JHT + WT B cells” vs “JHT + LTβ−/− B cells,” respectively. No significant difference was found in panels C and D between day 8 “WT + control Ig” and “JHT + WT B cells” nor between “WT + LTβR-Ig” and “JHT + LTβ−/− B cells” (1-way ANOVA). (E) Percentage of B-cell volume over total PLN volume in WT mice on day 0 (D0) or day 8 (D8) after infection after treatment of mice with control Ig or LTβR-Ig. ***P < .001. (F) Absolute B-cell number per inguinal LN in mice treated with control Ig or LTβR-Ig on day 8 after infection. n.s. indicates not significant. Samples were pooled from 3 to 9 PLNs from 2 to 8 mice in 2 independent experiments. Data are shown as mean ± SEM. (G) Average HEV segment length on day 0, 2, 5, and 8 after infection. ▭ indicate 20 to 100 μm;  , 100 to 200 μm; ▬, greater than 200 μm. On day 2, the proportion of segments less than 100 μm is significantly increased, whereas the proportion of segments greater than 200 μm is increased on day 8 after infection. Samples were pooled from a total of 4 to 5 inguinal LNs from 2 to 3 mice for each time point. (H) Total HEV segment number in WT and JHT mice infected with LCMV. Filled columns represent day 8 after infection. (I) Percentage of HEV with diameter greater than 50 μm during LCMV infection. Filled columns represent day 8 after infection. Data are shown as mean ± SEM. *P < .05, ***P < .001 compared with “D0” (1-way ANOVA).
, 100 to 200 μm; ▬, greater than 200 μm. On day 2, the proportion of segments less than 100 μm is significantly increased, whereas the proportion of segments greater than 200 μm is increased on day 8 after infection. Samples were pooled from a total of 4 to 5 inguinal LNs from 2 to 3 mice for each time point. (H) Total HEV segment number in WT and JHT mice infected with LCMV. Filled columns represent day 8 after infection. (I) Percentage of HEV with diameter greater than 50 μm during LCMV infection. Filled columns represent day 8 after infection. Data are shown as mean ± SEM. *P < .05, ***P < .001 compared with “D0” (1-way ANOVA).
LT signaling during LCMV-induced PLN remodeling. (A) Experimental layout for LT blocking in WT mice and B-cell reconstitution in JHT mice, followed by LCMV infection. WT mice received 2 injections on day 0 and 3 after infection of 100 μg of control or LTβR-Ig. JHT mice were reconstituted with WT or LTβ−/− B cells as described in Figure 4A. (B) Representative OPT images of inguinal LN isolated from day 8 (D8) LCMV-infected WT, WT + LTβR-Ig, JHT + WT B cells, and JHT + LTβ−/− B cells mice. Scale bar = 400 μm. (C) Inguinal LN volume on day 0 (D0) or day 8 (D8) after infection after treatment of mice with control Ig or LTβR-Ig or in JHT mice reconstituted with WT or LTβ−/− B cells. (D) Total HEV length on day 0 (D0) or day 8 (D8) after infection as in panel C. All day 8 values in panels C and D were significantly greater than on corresponding day 0 (Student t test). *P < .05, **P < .01, ***P < .001 comparing day 8 LCMV “WT + control Ig” vs “WT + LTβR-Ig” and “JHT + WT B cells” vs “JHT + LTβ−/− B cells,” respectively. No significant difference was found in panels C and D between day 8 “WT + control Ig” and “JHT + WT B cells” nor between “WT + LTβR-Ig” and “JHT + LTβ−/− B cells” (1-way ANOVA). (E) Percentage of B-cell volume over total PLN volume in WT mice on day 0 (D0) or day 8 (D8) after infection after treatment of mice with control Ig or LTβR-Ig. ***P < .001. (F) Absolute B-cell number per inguinal LN in mice treated with control Ig or LTβR-Ig on day 8 after infection. n.s. indicates not significant. Samples were pooled from 3 to 9 PLNs from 2 to 8 mice in 2 independent experiments. Data are shown as mean ± SEM. (G) Average HEV segment length on day 0, 2, 5, and 8 after infection. ▭ indicate 20 to 100 μm;  , 100 to 200 μm; ▬, greater than 200 μm. On day 2, the proportion of segments less than 100 μm is significantly increased, whereas the proportion of segments greater than 200 μm is increased on day 8 after infection. Samples were pooled from a total of 4 to 5 inguinal LNs from 2 to 3 mice for each time point. (H) Total HEV segment number in WT and JHT mice infected with LCMV. Filled columns represent day 8 after infection. (I) Percentage of HEV with diameter greater than 50 μm during LCMV infection. Filled columns represent day 8 after infection. Data are shown as mean ± SEM. *P < .05, ***P < .001 compared with “D0” (1-way ANOVA).
, 100 to 200 μm; ▬, greater than 200 μm. On day 2, the proportion of segments less than 100 μm is significantly increased, whereas the proportion of segments greater than 200 μm is increased on day 8 after infection. Samples were pooled from a total of 4 to 5 inguinal LNs from 2 to 3 mice for each time point. (H) Total HEV segment number in WT and JHT mice infected with LCMV. Filled columns represent day 8 after infection. (I) Percentage of HEV with diameter greater than 50 μm during LCMV infection. Filled columns represent day 8 after infection. Data are shown as mean ± SEM. *P < .05, ***P < .001 compared with “D0” (1-way ANOVA).
B cells are an important source of the membrane-bound LTα1β2, a ligand for LTβR.36 Because our results support a function for B cells and LTβR signaling in remodeling the PLN architecture during LCMV infection, we examined whether B cell–expressed LT was involved in this process. JHT recipient mice were reconstituted with LTβ−/− B cells on day −2 and 0 before LCMV infection and analyzed by OPT on day 0 and 8 after infection (Figure 6A). Although WT B cells were able to restore the expansion of PLN volume and HEV length, we observed a significant reduction in the total PLN volume in LTβ−/− B cell–reconstituted JHT mice, comparable with the one observed in LTβR-Ig–treated mice (Figure 6B-C). The total HEV length in LTβ−/− B cell–reconstituted JHT mice was also shorter compared with WT B cell–reconstituted PLN, although LTβR-Ig–treated mice were more compromised in the growth of the HEV network (Figure 6D). In conclusion, LTβ on B cells is critically involved in LCMV-induced PLN expansion after an initial, LTβ-independent B-cell accumulation in inflamed PLNs.
The endothelial surface available for lymphocyte recruitment can be accounted for through increased individual segment length, vasodilation, and/or increased branching. We exploited the optical information provided by OPT to directly examine these options. In the resting state, most HEV (61 ± 6%; mean ± SD) were between 20 and 100 μm length, 31 plus or minus 3% between 100 and 200 μm and 8 plus or minus 3% more than 200 μm. On day 2 after infection with LCMV, the proportion of segments shorter than 100 μm was increased compared with all other time points measured, whereas these PLN contained fewer segments longer than 200 μm than on day 5 and 8 after infection (Figure 6G). This finding is indicative of new branch formation from existing venules. In contrast, we observed a significant increase on day 8 after infection in HEV segments longer than 200 μm (P < .01; Figure 6G).
The most striking defect in absence of B cells and LT signaling was a strong reduction of virus-induced increase of HEV segments (Figure 6H). In JHT mice, this could be completely restored by transfer of B cells, whereas LTβ−/− B cells were able to support an increase of the HEV segment number to levels found on day 5 after infection of WT PLN (Figure 6H; V.K., J.V.S., unpublished data). Lack of B cells or LT signaling had a lesser effect on HEV diameter increase (Figure 6I) and did not impair the appearance of HEV segments greater than 200 μm (V.K., J.V.S., unpublished data). In summary, these data support a scenario in which B cell–induced HEV growth occurs in a first phase through increased branching and, at least in part, through elongation and circumferential growth of preexisting vascular segments at later time points (supplemental Figure 8).
Discussion
Here, we deliver a detailed analysis of the global microstructure of murine PLNs under homeostatic conditions in naive animals and inflammation-induced remodeling using quantitative mesoscopic imaging. Our data suggest a conserved homeostatic regulation of the basic internal PLN architecture. During LCMV infection, a threshold level of B cells was sufficient to induce vigorous PLN expansion, to a large extent through their surface expression of LTα1β2. In contrast, we could not find evidence for a role of VEGF-A during PLN remodeling.
Recent advances in microscopy have allowed important insights into the functioning of the immune system. In particular, 2PM has proven an invaluable tool in the direct visualization of lymphocyte dynamics during migration and interactions with APCs or other target cells in lymphoid and extralymphoid tissue.3,37-41 However, it is often desirable in experimental biology to obtain a global, comprehensive overview over structures too large for conventional microscopy yet too small for meaningful analysis by large-structure imaging techniques such as magnetic resonance imaging. Mesoscopic imaging approaches such as selective plane illumination microscopy and OPT have been developed to fill this “imaging gap” and thus permit qualitative and quantitative examination of anatomical structures in objects between 0.5 to 15 mm diameter, such as embryos and most mouse organs.42
A detailed mesoscopic analysis of the PLN architecture in antigen-naive animals revealed that irrespective of the PLN size, a constant ratio between PLN volume, B-cell volume, number of B-cell follicles, and HEV length is maintained. It has been shown that B-cell follicle formation depends on a positive feedback loop between LTα1β2-expressing B cells and LTβR-expressing FDC, in which LTβR signaling initiates secretion of CXCL13, which in turn attracts additional B cells to growing follicles.43 The global PLN imaging approach applied in this study supports the notion that this process is intrinsically self-limiting because follicles are typically size-restricted to less than 25 × 106 μm3 in homeostasis. How the homeostatic size is regulated on a molecular and cellular level is unclear at the moment but could be related to the number of B cells present in a given lymphoid organ. Thus, the average follicle size in B cell–rich PPs is larger than in B cell-poor PLNs.
An acute infection with LCMV is efficiently controlled by the cytotoxic T-cell response of the host,44,45 and B cells are not required for the initial control of low-dose LCMV infection.33,34 Hence, altered viral replication in B cell–deficient mice can be excluded as a reason for the different PLN restructuring observed in JHT mice. Here, we found that LCMV-induced growth of the HEV network but not their MECA-79+-phenotype depended to a large extent on LTα1β2 expression by B cells. Because general inhibition of LT signaling induced a more efficient reduction of PLN expansion, other LT-expressing cell types such as activated T cells, or lymphoid tissue inducer cells, which accumulate in SLOs during LCMV infection,17 might as well contribute to PLN remodeling during viral infection. Our data furthermore show that besides LT signaling, additional mechanisms control PLN growth. Monocyte subsets have been recently implicated in tumor angiogenesis.46,47 Similarly, we observed monocyte recruitment into LCMV-infected PLNs (R.D., J.V.S., unpublished data). It will be interesting to investigate a possible role for monocytes during PLN remodeling and a potential cross-talk with B cells.
B cells also were found to be required in nonviral inflammation-induced PLN expansion. In particular, the B cell–secreted proangiogenic factor VEGF-A acts as a central mediator for lymph angiogenesis and PLN growth,12 which may help to create a positive feedback loop through recruitment of increased numbers of DCs from the periphery, which in turn secrete angiogenic factors48 and have been implicated in PLN remodeling.14 In addition to the direct effect of B cell–secreted VEGF-A, LTα1β2 binding to LTβR induces VEGF-A secretion by FRCs.19 However, under the conditions used here, we were unable to uncover a major role for VEGF-A/VEGFR2 signaling during LCMV-induced PLN growth. The lack of increased VEGF-A or VEGFR2 expression and the inefficiency of anti-VEGF treatment during the course of an LCVM infection argue for a minor role of this pathway in this infection model. Therefore, the molecular mechanism by which LT signaling leads to PLN expansion during LCMV infections remains unclear. In preliminary experiments using 2-PM, we observed prolonged B-cell dwelling time around HEV of inflamed PLNs (R.D., J.V.S., unpublished data). We therefore favor a model in which direct molecular interactions between LTα1β2+ B cells and LTβR+ high endothelial cells during inflammation-induced recruitment of blood-borne B cells are blocked by the LTβR-Ig, although future experiments are needed to address whether B-cell behavior in and around HEV is related to LT signaling. Alternatively, LT signaling may contribute to the production of additional proangiogenic factors, such as FGF2.49,50
The molecular and cellular requirements for lymphoid tissue restructuring are likely to differ depending on the type of immune response. For example, although B cells are indispensable for PLN expansion in adjuvans- and LCMV-induced immune challenges, VEGF-A produced at peripheral sites induces B cell–independent lymphangiogenesis and PLN expansion.12,15 These different requirements may be linked to the source of morphogenic factors, that is, afferent lymph derived versus lymphoid tissue derived. Along the same line, our preliminary results indicate a different PLN remodeling pattern during infection with the cytopathic vesicular stomatitis virus. In this model, B-cell follicles increase in size but without a concomitant increase in PLN size or HEV network length (supplemental Figure 9), indicating that lymphoid tissue restructuring differs even between viral infections. Finally, the molecular and cellular mechanisms leading to the restoration of a normal PLN architecture have only begun become investigated. Both LTα and LTβR mRNA levels peak during an LCMV infection on day 4 after infection before rapidly decreasing to baseline levels by day 8 to 25.17 A future challenge will be to identify the roles for B cells, LT, and other factors during expansion and contraction of lymphoid tissue and thus to establish “rules of morphogenesis” directing the restructuring of lymphoid tissue and their interdependence with the outcome of immune response.
In summary, we identify LT on B cells and non-B cells as a major morphogenic factor governing PLN growth during LCMV infections. Furthermore, we provide evidence that mesoscopic imaging is a promising approach to describe in detail lymphoid microarchitecture in homeostasis and inflammation-induced changes. Clearly, many issues remain to be addressed, such as the precise mechanisms of vascular and lymph angiogenesis and the contribution of PLN stroma during early remodeling. We expect that mesoscopic imaging will contribute to a better understanding of the rules underlying morphologic changes in homeostasis and inflammation.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Prof Britta Engelhardt for continuous support, and Drs James Sharpe, Ulrich H. von Andrian, Tobias Junt, Mark Coles, and Urban Deutsch for critical reading of the manuscript and helpful suggestions. We are grateful to Dr Jeff Browning and Biogen Idec for their generous gift of anti-CD20 mAb and control and LTβR-Ig fusion protein.
Authorship
Contribution: V.K., E.S., R.D., L.O., and M.N. performed experiments; Y.F. provided important material; and V.K., E.S., C.H., B.L., and J.V.S. designed the research and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jens V. Stein, University of Bern, Theodor Kocher Institute, Freiestr 1, 3012 Bern, Switzerland; e-mail: jstein@tki.unibe.ch.

![Figure 1. Mesoscopic examination of naive PLN volume, B-cell follicle structure, and HEV network. (A) Workflow of OPT analysis of PLN architecture. PLN labeled with B220-Alexa488 and MECA-79-Alexa594 mAbs were dehydrated, cleared in BABB, and imaged in an OPT scanner as described in “Whole-mount staining for secondary lymphoid organs and preparation for OPT” (top). In some experiments, MECA-79-Alexa568 was used with similar results. After OPT image acquisition, samples can be further analyzed by high-resolution laser scanning microscopy (LSM) or rehydrated for flow cytometry analysis (bottom). As seen on the left (OPT), the current resolution clearly identifies HEV but not individual endothelial cells as seen when high-resolution LSM is used. Scale bar in LSM = 40 μm. (B) Schematic representation of primary OPT images (left; shown is 1 of 400 raw images before 3-dimensional [3D] reconstruction), 3D reconstruction (middle), and quantification of fluorescently labeled structures (right). Before OPT imaging, PLNs were whole-mount labeled with B220-Alexa488 (for B-cell follicles) and MECA-79-Alexa594 (anti-PNAd for HEV) mAbs and processed as described in “OPT scanning and software-based 3D reconstruction and quantification.” The length of the long axis of the inguinal PLN shown is 2.4 mm.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/115/23/10.1182_blood-2009-10-250118/4/m_zh89991051590001.jpeg?Expires=1770140734&Signature=t~VxklzzhIOQFp~CUgWmxs1TZXfkTvHjAzhhovBBbEdtjPMST6jFmRaKopRVeged2f-hJH10P9lMArAvv7CIwY2QXO5sxf2E8QyANqo8LuzNmN4FZhfVu2T-EOVQOybwYjFpCKpOfAPmtgbDzH9VtPdX1Sz4TByHmBZqORxfoIWMl9vT4p3j-2hjSf9f64nOFLsaJJxKl7ap0gflbuKNBsWNbnIAlLmQW9XSd8fbibSGF1hc0Wd0CEclCgLLnMNnLccqCxJuTmMBbOdquMLwCIgENfxb3Bo804aLb0kLKvFNI2bQTuUCoOSSkwMjJt0M9C9O~ZWUrShNMdg~x41UqQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal