In this issue of Blood, Coghill and colleagues reveal a critical role of lymphoid CCR7 for the initiation of acute GVHD.1 The authors report that CCR7 deficiency impairs the initiation of acute graft-versus-host but does not interfere with the desired antitumor effect. Can the interference with this important homing receptor bring the field of allo-HCT one step closer to achieving the ultimate goal of providing a targeted cellular anticancer therapy without the risk of GVHD?
A functioning immune system is highly dependent on interactions between specialized immune cells at the right time in the right place. For this reason, dynamic and coordinated immune cell migration throughout the body and within tissues is a prerequisite for efficient immunosurveillance and tolerance. Immune cell migration is orchestrated by the precise interplay of adhesion molecules and chemokines and their appropriate receptors, and has become the focus of attention in recent years due to the potential to modulate immune cell function for therapeutic purposes.
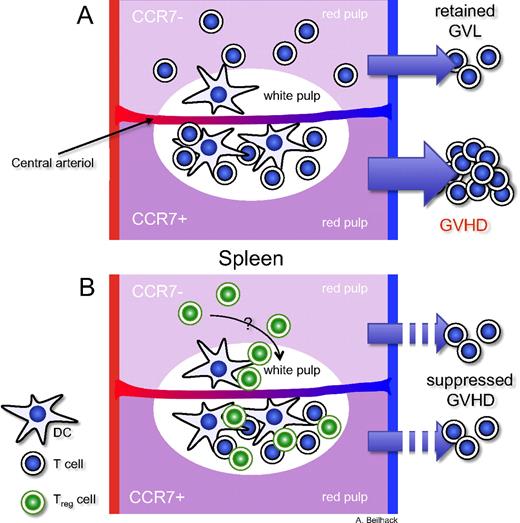
Critical role of CCR7 for the initiation of acute GVHD. The top halves of the schemes represent models of transplanted allogeneic CCR7-deficient T-cell subsets. In the bottom halves, allogeneic T cells express CCR7. (A) CCR7-deficient T cells cannot efficiently enter the T-cell zones where antigen presentation occurs. Instead, they accumulate in the red pulp. However, the antitumor effect is retained. (B) CCR7-deficient Treg cells can suppress alloreactive T cells in myeloablative conditioned allogeneic recipients as well as wild-type Treg cells.
Critical role of CCR7 for the initiation of acute GVHD. The top halves of the schemes represent models of transplanted allogeneic CCR7-deficient T-cell subsets. In the bottom halves, allogeneic T cells express CCR7. (A) CCR7-deficient T cells cannot efficiently enter the T-cell zones where antigen presentation occurs. Instead, they accumulate in the red pulp. However, the antitumor effect is retained. (B) CCR7-deficient Treg cells can suppress alloreactive T cells in myeloablative conditioned allogeneic recipients as well as wild-type Treg cells.
The interaction of the chemokines CCL19 and CCL21 with CC-chemokine receptor 7 (CCR7) on naive T cells and dendritic cells (DCs) guide these cells into the T-cell zones of lymph nodes, Peyer patches, and the spleen. In these defined areas, antigen presentation takes place and results in T-cell activation, proliferation, and differentiation during adaptive immune responses.2
It has been shown in different experimental settings that secondary lymphoid organs are essential sites for acute graft-versus-host disease (GVHD) initiation.3 Blocking T-cell access to lymph nodes and Peyer patches did not hinder alloreactive T-cell homing to the spleen. However, splenectomy of the recipient mice before allogeneic stem cell transplantation (allo-HCT) and subsequent treatment with blocking antibodies prevented GVHD in these mice. These proof-of-principle experiments could demonstrate the importance but also redundancy of lymph nodes, Peyer patches, and the spleen as initiation sites of acute GVHD.3 However, the described experimental set-up had been chosen for conceptual reasons rather than for direct clinical translation.
Coghill et al refined these experiments with an elegant approach of transplanting allogeneic CCR7-deficient T cells into myeloablative conditioned mice.1 They demonstrate that the CCR7-deficient T cells are defective in homing to lymph nodes and Peyer patches. Although they found allogeneic CCR7-deficient T cells accumulating in the spleen, these cells did not locate properly in the T-cell zones of the white pulp, where efficient antigen presentation takes place, but instead appeared predominantly in the red pulp. This resulted in reduced acute GVHD. These findings emphasize that alloreactive T cells need efficient access to the proper location within secondary lymphoid organs for the initiation of acute GVHD.
Next, the authors investigated whether CCR7-deficient T cells would be able to mount an efficient antileukemia response. They found that CCR7-deficient T cells cleared the tumor cells and prolonged survival of transplanted mice. As an explanation the authors suggest that limited numbers of activated T cells may be sufficient for an antitumor effect, whereas acute GVHD requires a massive expansion of alloreactive T cells. How this tumor cell killing takes place and where CCR7-deficient T cells are primed against tumor antigens or whether this is a reflection of a tamed but sufficient alloresponse against major histocompatibility complex (MHC)–mismatched leukemia cells remains to be investigated.
Is there a role of CCR7 in maintaining immunotolerance? CCR7 directs DCs and CD4+CD25+FoxP3+ regulatory T cells (Tregs) to proper locations within secondary lymphoid organs.4 Furthermore, CCR7 proved crucial for Treg function in experimental models like inflammatory bowel disease and contact hypersensitivity reactions because of their impaired homing to secondary lymphoid organs.4 Previously, it has been shown in allo-HCT that lymphoid-homing Treg cells in particular are capable of abrogating GVHD initiation.5 Thus, a clinically relevant question was to determine whether the lack of CCR7 on Tregs would impair their function in allo-HCT. The authors discovered that in the absence of CCR7, Treg-mediated suppression was not diminished and could prevent acute GVHD in vivo. In their experimental set-up, they had transferred CCR7-deficient T cells 2 days before the transfer of alloreactive wild-type T cells. It has been demonstrated that inflammatory conditions in lymphopenic hosts, as they exist in myeloablative transplanted allo-HCT recipients, provide stimuli to dramatically expand Tregs.6 The work by Coghill et al opens new doors for addressing further relevant questions: How could CCR7-deficient Tregs mediate their suppressive function in vivo? Which homing receptors besides CCR7 permitted Treg access to critical sites of alloreactive T-cell expansion? In which compartment did Tregs encounter alloreactive T cells to exert their down-modulatory potentials?
To simulate clinical allo-HCT, the authors performed transplantations in MHC-matched and haploidentical recipients and compared this to an experimental model of MHC fully mismatched transplantation. Importantly, in the MHC-matched and haploidentical transplantation setting, CCR7-deficient donor T cells caused less acute GVHD. Yet, in the MHC fully mismatched setting, the observed effect disappeared and mice developed acute GVHD, as had been previously described.1,7 A possible explanation for this important discrepancy between the different allo-HCT settings is that receptors other than CCR7 may be in play under aggravated inflammatory conditions, as they occur in MHC fully mismatched allo-HCT. Such observations of pathologic T-cell responses have been made in the absence of CCR7 in other models.2 For example, effector T cells can use a CCR7-independent pathway to enter lymphoid organs via the CXC chemokine receptor 3 (CXCR3).8 Thus, for clinical translation of the findings reported by Coghill et al, short-term blocking of CCR7 appears therapeutically appealing. Taking all data into consideration, it appears necessary to uncover additional homing pathways that direct T-cell subsets under severe inflammatory conditions. A future therapy interfering with alloreactive T-cell trafficking will most likely target a combination of relevant receptors and pathways.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal