Gross lymph node morphologic changes often accompany infections. In this issue of Blood, Kumar and colleagues show that B cells contribute to lymphoid remodeling via LTs following viral insults.1
A crucial function of the lymph node (LN) is to facilitate physical interactions among rare immune cells arriving from various tissue compartments. The unique positioning of the LN at the interface between the blood and lymphatic systems allows tissue-derived antigen and antigen-presenting cells (APCs) to congregate in close proximity to blood-derived lymphocytes. Increased lymphocyte recruitment, decreased egress, and enhanced cytokine/chemokine communication network, together with changes in LN architecture to accommodate the massive cellular influx, all conspire to accomplish one goal: to enhance physical encounters between relevant lymphocytes and APCs that lead to timely and efficient immune activation.2,3
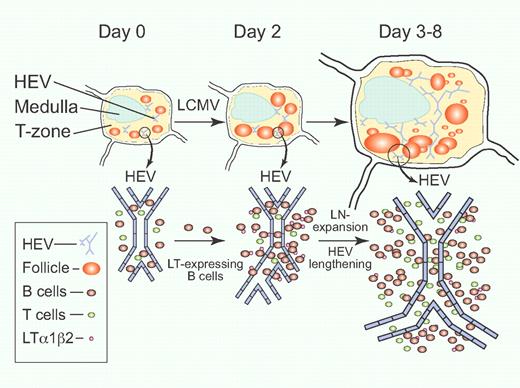
LTαβ-expressing B cells contribute to lymph node remodeling after LCMV infection. Circulating B cells are induced to express membrane-bound LTαβ upon entering the LN early during LCMV infection on day 0. A large influx of B cells occurs during the first 2 days, accompanied by increasing B-cell follicle size and HEV branch formation. Enlargement of LN size, lengthening of HEV, and new B-cell follicle formation then ensue from day 3 to day 8 after LCMV infection.1 Professional illustration by Paulette Dennis.
LTαβ-expressing B cells contribute to lymph node remodeling after LCMV infection. Circulating B cells are induced to express membrane-bound LTαβ upon entering the LN early during LCMV infection on day 0. A large influx of B cells occurs during the first 2 days, accompanied by increasing B-cell follicle size and HEV branch formation. Enlargement of LN size, lengthening of HEV, and new B-cell follicle formation then ensue from day 3 to day 8 after LCMV infection.1 Professional illustration by Paulette Dennis.
In recent years, there has been an increased appreciation of the role of B cells in lymphoid tissue organogenesis and immune response modulation. Specifically, B cell–derived lymphotoxin (LT) plays a vital role in the development of B-cell follicles, T-cell zones, follicular dendritic cells (FDC), splenic stromal cell subsets, and ectopic lymphoid tissue in autoimmune target organs.4-10 Studies have shown that LTs, together with tumor necrosis factor (TNF) and LIGHT (LT-related inducible ligand that competes for glycoprotein D binding to herpesvirus entry mediator on T cells), compose an integrated signaling network necessary for efficient innate and adaptive immune responses.11 There are 2 major forms of LT molecules with distinct receptor utilizations. While the homo-trimeric LTα3 is secreted as a soluble molecule and binds to TNFR1 and TNFR2 but not LTβR, only the membrane-bound hetero-trimeric LTα1β2 (LTαβ) binds to LTβR, which is constitutively present on stromal fibroblasts, epithelial cells, and myeloid cells. Cytokines (eg, IL-4, IL7) and chemokines (eg, CCL19, CCL21) can induce membrane-bound LTαβ on splenic T cells; however, most naive follicular B cells and naive CD4+ T cells constitutively express LTαβ. As they migrate to the blood, B cells rapidly lose LTαβ expression. Upon entering the LN, however, CXCR5+ B cells re-express membrane-bound LTαβ upon sensing a CXCL13 gradient produced by LTβR+ stromal cells. Direct stromal cell – B cell interaction through the LTαβ-LTβR axis further promotes production of CXCL13 by stromal cells, thereby completing the circuit.8,10 B-cell expression of LTαβ is further induced by CD40-CD40L interaction.12 The LTαβ-LTβR signaling therefore allows unidirectional communication between lymphocytes and surrounding stromal and parenchymal cells in a cell-cell contact-dependent manner.
Kumar et al investigated the global 3D LN structural remodeling phenomenon during infection with lymphocytic choriomeningitis virus (LCMV) by using a novel mesoscopic imaging tool, the optical projection tomography (OPT).1 This new imaging technique bridges the resolution gap between whole-animal imaging techniques (such as magnetic resonance imaging [MRI] and computed tomography [CT]) and microscopic confocal/ 2-photon cellular imaging modalities. The OPT technique captures static images of the whole-organ with resolution at the follicle and high endothelial venule (HEV) level, while allowing quantitative fluorescence analyses of structural data. Using this and other techniques, the authors provide evidence that LN enlargement and remodeling following LCMV infection is also dependent on the action of LTαβ-expressing B cells for the induction and maintenance of LN volume and HEV expansion (see figure). Transfer of wild-type B cells into B cell–deficient JHT recipient mice demonstrates a dose-dependency of B cells on LN growth. This LN expansion is independent of VEGF-A, as administration of a VEGFR inhibitor, sunitinib, and anti-VEGFR2/anti-VEGF-A neutralizing antibodies did not alter LN growth in the LCMV infection model. Interestingly, although B cells constitute a major source of LT, approximately 30% of post-LCMV LN enlargement and HEV lengthening is unaccounted for by B cells alone. Thus, the search for other sources of LT continues.
Unresolved questions remain to fully depict the process of infection-related LN remodeling. The precise LT-mediated molecular and cellular network responsible for inducing LN growth needs further study. Scandella et al showed that LN mRNA levels of LTα and LTβR were elevated early in LCMV infection.13 As LTβR is expressed by stromal cells including HEV and FRC, cross-linking LTβR by LT has been shown to induce VEGF-A expression. Although the current data presented by Kumar et al do not support a role for VEGF-A in LN enlargement and HEV growth in LCMV infection, its role in LN enlargement associated with other infections needs to be clarified. The role of T cells, particularly constitutive LT-expressing CD4+ T cells also needs to be further defined. The use of inducible, tissue-restricted LT-deficient animal models will help elucidate these issues. It is yet to be shown whether the dependence on LT and B cells is a general phenomenon in other non-LCMV infection-associated lymphoid remodeling processes. Indeed, the authors provide evidence suggesting that VSV-associated LN remodeling is not accompanied by HEV lengthening or changes in total LN volume.1 It is therefore likely that the molecular mechanisms reported by Kumar et al do not fully describe all the molecular and cellular rules of LN morphogenesis.
The authors in the current study observed an association between HEV length and the absolute number of lymphocyte recruitment in the LN. This implies a direct correlation between the length of the HEV to the number of available port-of-entry sites for lymphocyte extravasations from general circulation.14 This raises the debate of whether specific HEV “hot spots” truly exist for lymphocyte extravasations, and future investigation will be needed to resolve this issue. Additionally, Bajenoff and Germain recently reported that the remodeling of the conduit system that allows efficient delivery of soluble antigens from the periphery to FDC accompanies B-cell follicle development. It remains to be determined what role, if any, LTαβ expression by B cell may contribute to this process.15
Finally, the observation presented in this report also poses the possibility that a similar paradigm may exist to accomplish LN resolution following viral clearance. As mRNA levels of LTα and LTβ diminish after day 4 after LCMV infection, there is a concomitant decrease in the number of B cells in the LN.13 Understanding molecular and cellular identities involved in the restoration of LN architecture and lymphocyte homeostasis following viral insults will add to our knowledge base in understanding the complex communication network regulating immune responses in vivo. The current work by Kumar et al furthers our understanding in a rapidly evolving cutting-edge field and highlights the symbiotic relationship between anatomy and function in immunobiology.1
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal