Abstract
The sequential events leading to tumor progression include a switch to the angiogenic phenotype, dependent on a shift in the balance between positive and negative angiogenic regulators produced by tumor and stromal cells. Although the biologic properties of many angiogenesis regulatory proteins have been studied in detail, the mechanisms of their transport and delivery in vivo during pathologic angiogenesis are not well understood. Here, we demonstrate that expression of one of the most potent angiogenesis inhibitors, thrombospondin-1, is up-regulated in the platelets of tumor-bearing mice. We establish that this up-regulation is a consequence of both increased levels of thrombospondin-1 mRNA in megakaryocytes, as well as increased numbers of megakaryocytes in the bone marrow of tumor-bearing mice. Through the use of mouse tumor models and bone marrow transplantations, we show that platelet-derived thrombospondin-1 is a critical negative regulator during the early stages of tumor angiogenesis. Collectively, our data suggest that the production and delivery of the endogenous angiogenesis inhibitor thrombospondin-1 by platelets may be a critical host response to suppress tumor growth through inhibiting tumor angiogenesis. Further, this work implicates the use of thrombospondin-1 levels in platelets as an indicator of tumor growth and regression.
Introduction
Tumor growth beyond 1 to 2 mm3 requires the development of new blood vessels, a process known as angiogenesis.1 Tumor angiogenesis is triggered by proangiogenic regulators, such as vascular endothelial growth factor (VEGF), fibroblast growth factor, and platelet-derived growth factor, among others. Host defense against tumor angiogenesis comes in the form of endogenous angiogenesis inhibitors, such as thrombospondin-1 (TSP-1) and endostatin, which counteract the activity of proangiogenic factors and prevent in situ carcinomas from undergoing a switch to the angiogenic phenotype.2,3 The angiogenic response of the host is associated with the increase in the expression of proangiogenic proteins that are released from both tumor cells and stromal cells, such as fibroblasts and platelets.
Armand Trousseau made the original connection between platelets and cancer in 1865; however, the role of platelets in tumor angiogenesis remains unclear.4,5 Although platelets circulate throughout the body and participate in the control of hemostasis, increased platelet counts and activation of the coagulation cascade are often seen in cancer patients.6,7 In addition, several studies indicate a role for platelets in promoting metastatic disease. These studies using mouse models of metastasis demonstrated that induction of thrombocytopenia, with antiplatelet antibodies or by injecting neuraminidase before tumor cell inoculation, significantly reduced the formation and overall number of lung metastases.8-10 Furthermore, animals with dysfunctional platelet receptors, and Nf-e2−/− mice, which have virtually no circulating platelets, exhibited a 15-fold reduction in lung metastases or were completely protected against hematogenous metastasis, respectively.11,12
One of the most interesting characteristics of platelets is the large number of biologically active molecules carried in their granules, which can be deposited at sites of vascular injury after platelet activation. Although platelets are anuclear and lack most of the machinery necessary for protein production, platelets contain a large number of proangiogenic and antiangiogenic regulators, the majority of which are packaged into α granules.5,6 By adhering to the endothelium of injured tissues and then secreting their granular contents, platelets deposit high concentrations of angiogenesis-regulatory proteins in a regulated and localized manner. Tumor cells are also known to promote platelet activation and aggregation by the release of platelet-activating factors, such as thrombin and adenosine diphosphate,13 resulting in the release of α granules and their content, which in turn directly affects tumor growth.14 Recently, we have described the storage of proangiogenic and antiangiogenic factors within separate platelet α granules,5 whereas others have demonstrated selective release of either proangiogenic or antiangiogenic proteins on platelet activation.15
One protein released during platelet activation is TSP-1, a potent antiangiogenic glycoprotein that constitutes as much as 20% of the total human platelet α granule content.16 In addition to platelets, several cell types express TSP-1, including stromal fibroblasts, endothelial cells, immune cells, and even some tumor cells, and its expression is strongly correlated to wound healing, tissue remodeling, and tumor growth.17-20 TSP-1 has been shown to inhibit migration and proliferation and induce apoptosis of endothelial cells.21 TSP-1 also directly affects megakaryopoiesis and the overall vascularity of the bone marrow.22-24 Despite remarkable progress over the past 25 years in understanding TSP-1 functions, the diagnostic and therapeutic potential of this molecule is not completely understood.
Recently, the release of platelet TSP-1 has been shown to inhibit thrombopoiesis, diminish bone marrow microvascular reconstruction after myelosuppression, and limit the extent of revascularization in a mouse model of hind limb ischemia, implying that Tsp-1–deficient thrombopoietic cells may function as proangiogenic agents.24 If TSP-1 in circulating platelets has a direct effect on tumor angiogenesis, the ability to selectively modulate the platelet α-granule TSP-1 content may assume considerable therapeutic importance. However, the direct effect that platelet TSP-1 has on tumor progression, as well as the mechanism of regulation and delivery of TSP-1 to the site of a growing angiogenic tumor, needs to be further investigated.
Methods
Animals
All animal experiments were performed according to protocols approved by Children's Hospital Institutional Animal Care and Use Committee. C57Bl/6 animals were purchased from Charles River. Tsp-1−/− mice were provided by Dr Jack Lawler (Beth Israel Deaconess Medical Center, Boston, MA).25 LSL-K-rasG12D x p53fl/fl mice were provided by Dr Carla Kim (Children's Hospital, Boston, MA).
Total body irradiation and bone marrow transplantation
Six-week-old Tsp-1−/− mice on a C57Bl/6 background were irradiated with a lethal dose of 9.5 Gy from a 137Cs-ray source at a dose of 0.80 Gy/minute. Irradiated mice were immediately reconstituted with donor bone marrow cells via tail vein injection. Donor bone marrow cell suspensions were isolated by flushing sterile phosphate-buffered saline into the femurs of 6-week-old wild-type or Tsp-1−/− mice on a C57Bl/6 background. Single-cell suspensions were prepared by passing the cells through a 30-μm filter, centrifuged, and resuspended at a concentration of 107 cells/100 μL. Mice engrafted with saline alone died within 1 week, whereas successful engraftment was monitored by survival, circulating platelet numbers, and expression of TSP-1 in platelets. Platelet TSP-1 expression was assessed by Western blot 6 weeks after engraftment in Tsp-1−/− mice and demonstrated comparable levels of platelet TSP-1 expression in most mice engrafted with wild-type bone marrow (supplemental Figure 2, available on the Blood Web site; see the Supplemental Materials link at the top of the online article) compared with wild-type mice.
Migration assay
Cell migration assays were performed using modified Boyden chambers (Transwell-Costar) coated with 10 mg/mL fibronectin as described previously.21 Platelets were collected by retro-orbital bleed using heparinized capillary tubes and isolated as previously described.5 Resting platelets from wild-type and Tsp-1−/− age-matched animals were activated by the addition of 1 unit of thrombin (Sigma-Aldrich) and incubated for 20 minutes at 37°C, followed by centrifugation for 5 minutes at 900g at room temperature. Platelet releasate was then transferred into a fresh tube and then added to the endothelial cells used in the migration assay.
Cell culture
Lewis lung carcinoma and B16F10 mouse melanoma cell lines were obtained from ATCC. All cells were maintained in Dulbecco modified Eagle medium (Lonza) supplemented with 10% fetal calf serum (Lonza), 100 U/mL penicillin (Lonza), and 100 μg/mL streptomycin (Lonza) at 37°C at 5% CO2.
Megakaryocyte isolation and culture
To obtain bone marrow–derived megakaryoblasts, femurs were dissected from Tsp-1−/− mice and the bone marrow was flushed with saline. Single-cell suspensions were generated by passage through a 100μM mesh and then cultured in Dulbecco modified Eagle medium supplemented with 10% fetal bovine serum and 25 ng/mL thrombopoietin for 3 days. Cultures were overlaid onto a discontinuous bovine albumin density gradient (0%/1.5%/3.0% in phosphate-buffered saline [PBS]) to obtain enriched populations of megakaryocytes.
Platelet isolation
Blood (∼ 900 μL) was collected from anesthetized mice by retro-orbital bleeding with heparinized capillary tubes (Fisher Scientific) into 0.1 volume of Aester-Jandl anticoagulant (85mM Na citrate, 69mM citric acid, 20 g/L glucose, pH 4.6) and centrifuged at 1.5g at room temperature for 8 minutes. The upper phase (∼ 400 μL), platelet-rich plasma, which contains platelets and platelet fragments, was transferred into a fresh tube, and platelets were separated by a second centrifugation step at 0.8g for 6 minutes. Platelets were pelleted by a final centrifugation step at 3.1g for 5 minutes.
Tumor growth
Six- to 8-week-old mice were inoculated with 106 tumor cells resuspended in 100 μL of PBS by subcutaneous or intraperitoneal injection. Mice were killed 10 to 14 days after tumor cell inoculation. Tumors were excised, weighed, and fixed.
Adeno-Cre infection
Mice were infected with 5 × 105 units (pfu) of adenovirus Cre recombinase (Adeno-Cre) virus at 6 to 8 weeks of age. Adeno-Cre/CaPi (Calcium Phosphate complex [precipitate]) coprecipitates were prepared as described.26 Mice were anesthetized with isoflurane. Adeno-Cre/CaPi coprecipitates were administered through intramuscular injection.
Quantification of TSP-1 mRNA
Total RNA was isolated from bone marrow megakaryocytes using TRIzol reagent (Invitrogen) according to the manufacturer's protocol and treated with DNaseI (NEB). RNA samples (1 μg) were reverse transcribed using Invitrogen Superscript III reverse transcriptase according to the manufacturer's instructions. Amplification was performed in a volume of 25 μL containing 8 μL of template cDNA, 10 μL of real-time DyNAmo HS SYBR Green master mix (Finzymes Inc), 5 μL of H2O, and 2 μL RT primer set. Amplification was performed for 40 cycles (95°C for 15 seconds, 60°C for 1 minute) on the DNA Engine Opticon 2 System (MJ Reseach Inc). The following primers were used: Tsp-1, sense: 5′- TCC CCT ATT CTG GAG GGT TC-3′; antisense: 5′-TCC CTG GAA ATA GGC ACA AG-3′; GAPDH, sense: 5′-ACC ACA GTC CAT GCC ATC AC-3′, antisense: 5′-TCC ACC ACC CTG TTG CTG TA-3′. For data analysis, the 2–DDCT method27 was used with normalization of raw data to the housekeeping gene GAPDH.
Western blot analysis
Platelets were isolated by retro-orbital bleed from wild-type and Tsp-1−/− mice as previously described and lysed in RIPAbuffer (Thermo Scientific) supplemented with protease inhibitor cocktail (Roche Diagnostics), 10mM NaF (Sigma-Aldrich), 1mM Na3VO4 (Sigma-Aldrich), and 1mM DTT (Sigma-Aldrich). Total protein concentration was quantified and equal amounts of lysates were loaded, separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and transferred onto nitrocellulose. Membranes were incubated overnight at 4°C with the primary antibodies as follows: TSP-1 (clone Ab-11; Lab Vision) diluted at 1:2000, VEGF (clone SC-152, Santa Cruz Biotechnology) diluted at 1:500, and β-actin (Sigma-Aldrich) at 1:5000. Subsequently, membranes were incubated with either goat anti–rabbit or anti–mouse antibodies conjugated to horseradish peroxidase (GE Healthcare), and protein was visualized by enhanced chemiluminescence system (ECL kit; GE Healthcare).
Immunohistochemistry
Formalin-fixed, paraffin-embedded tumors or femur sections were deparaffinized by successive incubations in xylene, 100% ethanol, 90% ethanol, and 70% ethanol followed by PBS. Epitopes were unmasked with 10 μg/mL proteinase K (Roche Diagnostics) in PBS at 37°C for 40 minutes and rinsed twice in PBS with 0.3% Triton X-100 (PBS-T). Sections were immunostained overnight at room temperature by incubation with rat anti-CD31 (BD Biosciences PharMingen) diluted at 1:50, or anti–mouse-TSP-1 Ab-4 (Lab Vision) diluted at 1:500 followed by incubation for 1 hour with goat anti–rat or goat anti–mouse Alexa 594–conjugated secondary antibody (1:500; Invitrogen). Nuclei were stained with 1% Hoechst dye for 1 minute.
Isolated platelets and megakaryocytes were fixed with 4% formaldehyde for 20 minutes, attached to poly-lysine–coated coverslips and processed for immunofluorescence microscopy. For bone marrow smears, femurs were dissected from mice and bone marrow flushed into a 12-well tissue culture dish.
Bone marrow cells were resuspended in saline and placed in a cytospin with 0.2% gelatin-coated slides. Cells were fixed with 4% paraformaldehyde for 10 minutes at room temperature followed by blocking in 3% milk in TBS-T for 30 minutes. Anti–TSP-1 mAb (clone Ab-4, Neomarkers) was added for 1 hour at room temperature. Cells were washed with PBS-T before the addition of rabbit anti–mouse-Alexa 594 (1:500; Invitrogen) for 30 minutes at room temperature protected from light. Nuclei were stained with 1% Hoechst dye for 1 minute. Cells were extensively washed with PBS-T, mounted, viewed, and photographed on a fluorescent microscope.
Histologic image acquisition and analyses
Images of bone marrow sections and tumors were taken with a digital camera (AxioCAM HRc, Carl Zeiss) mounted on Zeiss Imager M1 Axio. Plan-Neofluor 10×/0.25, 25×/0.50, and 40×/0.75 objective lenses were used. Images were recorded using Zeiss AxioVision Acquisition software (Version 4.5). Images of platelets and megakaryocytes were taken with Nikon TE 2000 Eclipse microscope equipped with a Nikon 100×/1.4 NA objective and a 100-W mercury lamp. Images were acquired with a Hamamatsu Orca IIER CCD camera. Electronic shutters and image acquisition were under the control of Molecular Devices Metamorph software. Images were acquired by fluorescence microscopy with an image capture time of 200 to 500 ms.
rTSP-1 treatment
Recombinant human TSP-1 protein (rTSP-1) was purchased from Protein Sciences Corporation. To determine the appropriate concentration of rTSP-1 for our studies, we delivered a range of rTSP-1 (0-10 μg) to Tsp-1−/− mice via tail vein injection. Platelets were isolated by retro-orbital bleed 5 days after TSP-1 treatment and probed for TSP-1 expression. Levels of TSP-1 in platelet lysates correlated with increasing doses of rTSP-1 delivery. A total of 2 μg of rTSP-1 was determined to be the lowest concentration of TSP-1 detected in platelets; therefore, this concentration was chosen for further experiments.
Platelets from Tsp-1−/− mice were isolated by retro-orbital bleed, resuspended in 1× platelet buffer (10×: 1.45M NaCl, 0.1M N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, 5.83mM NaH2PO4, 50mM KCl, 20mM MgCl2, pH 7.4), and incubated with rTSP-1 for 2 hours at 37°C. Platelets were then extensively washed before being analyzed by either immunohistochemistry or Western blot analysis.
Results
TSP-1 expression is increased in circulating platelets of tumor-bearing mice
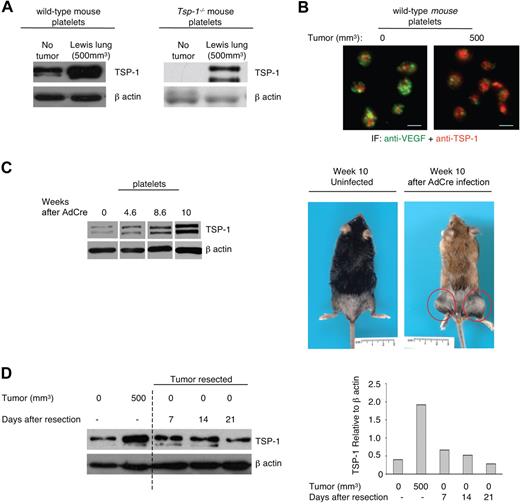
It has been previously hypothesized and more recently demonstrated that circulating platelets act as scavengers and sequester specific angiogenic regulators.28-30 To determine whether TSP-1 expression levels in platelets were a direct consequence of a growing tumor, we inoculated both wild-type and Tsp-1−/− mice with Lewis lung carcinoma tumor cells that express TSP-1 and B16F10 melanoma cells that do not express TSP-1. Platelets were collected from tumor-bearing animals when tumors reached 500 mm3. Western blot analysis of platelets isolated from both wild-type and Tsp-1−/− tumor-bearing and non–tumor-bearing mice showed increased TSP-1 expression in the presence of tumors that did (Figure 1A; supplemental Figure 1A) and did not express TSP-1 (supplemental Figure 1C).
TSP-1 expression in platelets is an indicator of tumor growth and regression. (A) Western blot analysis of TSP-1 expression in equivalent numbers of platelets harvested from wild-type and Tsp-1−/− mice immediately after inoculation (No tumor) with Lewis lung cells that express TSP-1 and in mice with tumor volumes of 500 mm3. β-Actin was probed as a loading control. (B) Immunofluorescence analysis of TSP-1 (red) and VEGF (green) expression in platelets harvested from wild-type mice before tumor growth (0) and after tumors reached 500 mm3 (500). Platelets were immunostained with secondary antibody alone to ensure specificity (data not shown). Bar represents 2 μm. (C) Western blot analysis of TSP-1 expression in platelets isolated from an inducible tumor model, LSL-K-rasG12D x p53fl/fl mice, after Adeno-Cre-induced sarcoma formation in the extremities. β-Actin was probed as a loading control. Right: Images of LSL-K-rasG12D x p53fl/fl mice that were mock infected (uninfected) and 10 weeks after Adeno-Cre infection. Red circles represent sarcomas. (D) Platelet TSP-1 expression by Western blot is probed during tumor growth and after tumor resection on the indicated days. β-Actin was probed as a loading control. Right: Quantification of TSP-1 expression relative to β-actin.
TSP-1 expression in platelets is an indicator of tumor growth and regression. (A) Western blot analysis of TSP-1 expression in equivalent numbers of platelets harvested from wild-type and Tsp-1−/− mice immediately after inoculation (No tumor) with Lewis lung cells that express TSP-1 and in mice with tumor volumes of 500 mm3. β-Actin was probed as a loading control. (B) Immunofluorescence analysis of TSP-1 (red) and VEGF (green) expression in platelets harvested from wild-type mice before tumor growth (0) and after tumors reached 500 mm3 (500). Platelets were immunostained with secondary antibody alone to ensure specificity (data not shown). Bar represents 2 μm. (C) Western blot analysis of TSP-1 expression in platelets isolated from an inducible tumor model, LSL-K-rasG12D x p53fl/fl mice, after Adeno-Cre-induced sarcoma formation in the extremities. β-Actin was probed as a loading control. Right: Images of LSL-K-rasG12D x p53fl/fl mice that were mock infected (uninfected) and 10 weeks after Adeno-Cre infection. Red circles represent sarcomas. (D) Platelet TSP-1 expression by Western blot is probed during tumor growth and after tumor resection on the indicated days. β-Actin was probed as a loading control. Right: Quantification of TSP-1 expression relative to β-actin.
Because we have previously demonstrated that proangiogenic and antiangiogenic regulators are stored in separate granules in platelets,5 we examined the expression of TSP-1 in platelets harvested from tumor-bearing and non–tumor-bearing wild-type mice by immunofluorescence (Figure 1B). Although the platelet VEGF levels remained unchanged (Figure 1B; supplemental Figure 1B), the number of TSP-1–reactive granules increased in the presence of a tumor (Figure 1B).
To further confirm the up-regulation of TSP-1 expression in platelets during tumorigenesis, we used an inducible tumor model. Sarcomas were induced in the LSL-K-rasG12D x p53fl/fl mice by intramuscular injection of Adeno-Cre.26 Platelets were collected every 2 weeks from mice injected with and without Adeno-Cre until tumors reached a volume of 300 to 500 mm3. Western blot analysis of platelets from tumor-bearing animals demonstrated a significant increase in TSP-1 levels as early as 4.6 weeks after infection (Figure 1C), further supporting the correlation between platelet TSP-1 levels and tumor size.
TSP-1 expression in platelets decreases after tumor resection
To investigate whether TSP-1 expression in platelets would also reflect tumor regression, we examined TSP-1 expression in platelets after tumor resection (Figure 1D). Wild-type mice were inoculated with Lewis lung carcinoma cells and tumors were resected after reaching approximately 500 mm3 in volume. Platelets were harvested from mice at various days after tumor resection, and platelet lysates were evaluated for TSP-1 expression. Twenty-one days after tumor resection, the level of TSP-1 in platelets of wild-type mice returned to baseline (Figure 1D). The observed initial up-regulation of TSP-1 in the presence of a growing tumor and the subsequent reduction in platelet TSP-1 levels on tumor resection implicate the potential of platelet TSP-1 as a biomarker of tumor growth and regression.
Platelet-derived TSP-1 delays tumor growth by suppressing angiogenesis
To identify the contribution of platelet-derived TSP-1 to tumorigenesis, we performed bone marrow transplantations. We transplanted into Tsp-1−/− mice either wild-type or Tsp-1−/− bone marrow; and after confirming that platelets from wild-type bone marrow recipients expressed TSP-1 (supplemental Figure 2A), we inoculated mice subcutaneously with Lewis lung carcinoma cells and monitored tumor growth (Figure 2; supplemental Figure 2B). Mice with circulating platelets lacking TSP-1 developed tumors 4 to 6 days earlier (Figure 2A). Because TSP-1 is a potent angiogenic inhibitor, we quantified tumor angiogenesis in tumors isolated from these mice. These data demonstrated that tumors isolated from mice with Tsp-1 null platelets had significantly higher microvessel density than tumors isolated from mice with wild-type platelets (Figure 2B).
Loss of TSP-1 in platelets leads to accelerated tumor growth and increased tumor angiogenesis. (A) Lewis lung tumor cells were inoculated in the flank, and tumor growth was measured by calipers at the indicated days in lethally irradiated Tsp-1−/− mice transplanted with either Tsp-1−/− bone marrow (green line; n = 5) or wild-type bone marrow (red line; n = 5). (B) Microvessel density (MVD) per high-power field (hpf) was quantified in equivalent volume tumors harvested from Tsp-1−/− mice transplanted with wild-type or Tsp-1−/− bone marrow (P < .005). (C) Bioluminescence images of intraperitoneal tumors in mice after injections of platelet buffer alone, 109 wild-type, or Tsp-1−/− platelets. (D) CD31 and TSP-1 immunofluorescence of tumors harvested from mice injected with platelet buffer alone (control), wild-type platelets, or Tsp-1−/− platelets. Tumors were immunostained with an antibody against CD31 (left panels) to detect endothelial cells and for TSP-1 (right panels). Bar represents 20 μm. (E) MVD/hpf was quantified by CD31 immunostaining of intraperitoneal tumors harvested from mice injected with platelet buffer, wild-type platelets, or Tsp-1−/− platelets (P < .005).
Loss of TSP-1 in platelets leads to accelerated tumor growth and increased tumor angiogenesis. (A) Lewis lung tumor cells were inoculated in the flank, and tumor growth was measured by calipers at the indicated days in lethally irradiated Tsp-1−/− mice transplanted with either Tsp-1−/− bone marrow (green line; n = 5) or wild-type bone marrow (red line; n = 5). (B) Microvessel density (MVD) per high-power field (hpf) was quantified in equivalent volume tumors harvested from Tsp-1−/− mice transplanted with wild-type or Tsp-1−/− bone marrow (P < .005). (C) Bioluminescence images of intraperitoneal tumors in mice after injections of platelet buffer alone, 109 wild-type, or Tsp-1−/− platelets. (D) CD31 and TSP-1 immunofluorescence of tumors harvested from mice injected with platelet buffer alone (control), wild-type platelets, or Tsp-1−/− platelets. Tumors were immunostained with an antibody against CD31 (left panels) to detect endothelial cells and for TSP-1 (right panels). Bar represents 20 μm. (E) MVD/hpf was quantified by CD31 immunostaining of intraperitoneal tumors harvested from mice injected with platelet buffer, wild-type platelets, or Tsp-1−/− platelets (P < .005).
Tumor cells have been shown to promote platelet activation and aggregation by the release of platelet-activating factors, such as thrombin and adenosine diphosphate.31 To directly examine the effects of platelet TSP-1 on tumor growth, we inoculated wild-type mice intraperitoneally with luciferase-labeled Lewis lung carcinoma cells that did not express TSP-1. Subsequently, platelets were harvested from either wild-type or Tsp-1−/− mice and were also directly injected into the intraperitoneal cavity with tumor growth monitored by bioluminescence imaging. Previous studies of endotoxin clearance by platelets demonstrated that intraperitoneal injection of platelets does not result in immediate platelet activation.32 Thus, we hypothesized that only direct contact with tumor cells would lead to local deposit of angiogenic regulators from the injected platelets. Because the life span of circulating mouse platelets has been shown to be 4.5 days,33 we began to monitor tumor growth beginning 72 hours after platelet injection and every day afterward. We observed significant suppression of tumor growth in mice injected with wild-type platelets relative to littermate controls injected with platelet buffer alone at 5 days after tumor cell inoculation (Figure 2C). Correspondingly, we observed increased tumor volume in the mice injected with Tsp-1−/− platelets as measured by bioluminescence (Figure 2C) and by tumor weight (supplemental Figure 3A). These data confirm that tumors treated with Tsp-1−/− platelets were significantly larger than those from mice treated with wild-type platelets. To ensure that the inoculated platelets went directly to the tumor site, we also injected green fluorescent protein (GFP)–labeled platelets and examined tumors and other organs for GFP expression by immunofluorescence. Although we did not detect GFP present on the liver surface, tumors were coated with GFP+ platelets (supplemental Figure 3B). Using anti-CD31 immunofluorescence to detect endothelial cells, we assessed tumor angiogenesis after treatment with wild-type versus Tsp-1−/− platelets (Figure 2D-E). Tumors from mice injected with wild-type platelets demonstrated significantly decreased microvessel density and high TSP-1 expression. In contrast, tumors from mice injected with Tsp-1-null platelets had increased microvessel density and negligible TSP-1 expression (Figure 2D-E). These data further support the notion that the loss of TSP-1 converts platelets into proangiogenic regulators.
TSP-1 expression in platelets is critical for modulating its overall angiogenic output
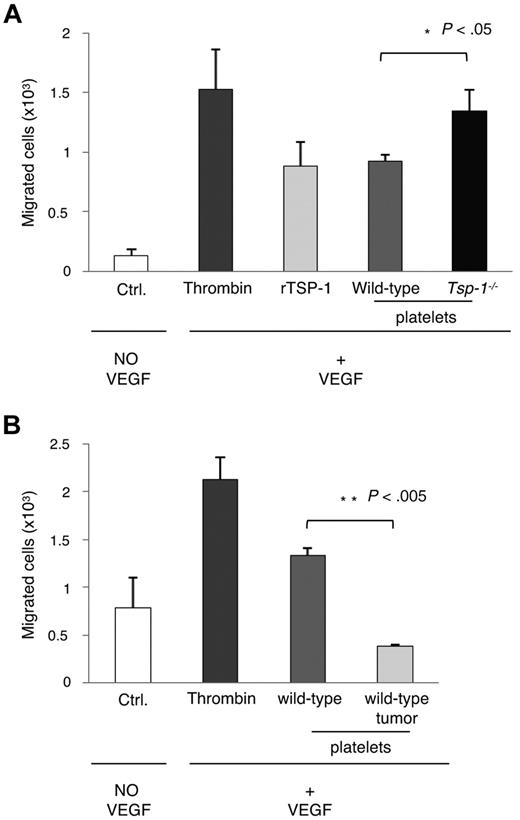
It has been previously suggested that platelets may be involved in negative regulation of angiogenesis.34 To examine the angiogenic behavior of platelets and the role of TSP-1, we examined the effect of the entire contents of platelets on endothelial cell migration, a functional assessment of endothelial activation and a prerequisite of angiogenesis. Platelets from wild-type and Tsp-1−/− mice were isolated, activated with thrombin, and the contents were added to endothelial cells. VEGF-induced migration was significantly inhibited in the presence of wild-type platelet contents compared with the contents from Tsp-1−/− platelets (Figure 3A; P < .05) and more significantly than treatment with rTSP-1. These data suggest that the loss of TSP-1 expression from platelets abrogated its antiangiogenic effect.
Endothelial cell migration increases in the presence of Tsp-1−/− platelet lysates. (A) Endothelial migration was measured in the absence of VEGF (Ctrl.) or in response to VEGF (5 ng/mL) in the presence of thrombin, rTSP-1, or platelet lysates from wild-type or Tsp-1−/− platelets activated by thrombin (P = .03). (B) Endothelial cell migration was measured in the absence of VEGF (Ctrl.) or in response to VEGF (5 ng/mL) in the presence of thrombin, or thrombin-treated platelet lysates from wild-type mice or tumor-bearing (500 mm3) wild-type mice (P = .001).
Endothelial cell migration increases in the presence of Tsp-1−/− platelet lysates. (A) Endothelial migration was measured in the absence of VEGF (Ctrl.) or in response to VEGF (5 ng/mL) in the presence of thrombin, rTSP-1, or platelet lysates from wild-type or Tsp-1−/− platelets activated by thrombin (P = .03). (B) Endothelial cell migration was measured in the absence of VEGF (Ctrl.) or in response to VEGF (5 ng/mL) in the presence of thrombin, or thrombin-treated platelet lysates from wild-type mice or tumor-bearing (500 mm3) wild-type mice (P = .001).
Because it has been previously demonstrated that platelets are able to scavenge both proangiogenic and antiangiogenic regulators,30 we wanted to determine the platelet angiogenic effect in tumor-bearing animals. Once again, we performed a migration assay using platelets isolated from tumor-bearing mice and observed a strong inhibitory effect of the platelet releasate on endothelial cell migration (Figure 3B). The increased levels of TSP-1 in platelets from tumor-bearing mice further amplified the inhibition of endothelial cell migration.
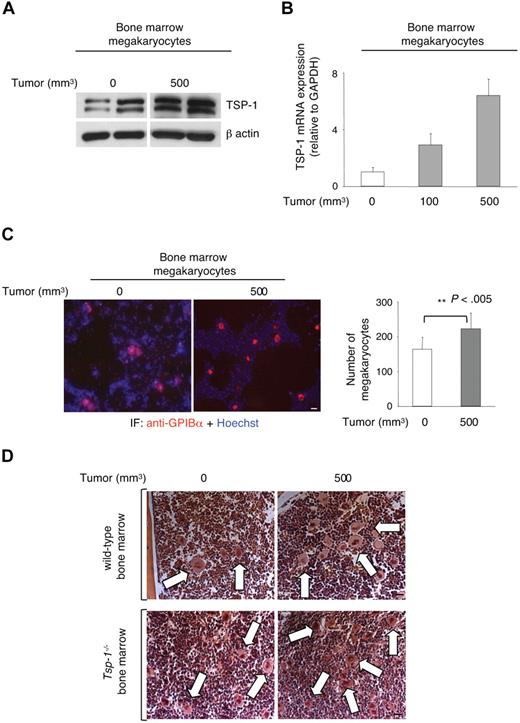
Megakaryopoiesis and TSP-1 synthesis in megakaryocytes are up-regulated during tumor growth
Although our studies suggest that megakaryocytes may directly endocytose TSP-1, it is also possible that the presence of a tumor may stimulate megakaryocytes to synthesize more TSP-1. Almost all the proteins within platelet α granules are produced and packaged during platelet formation and before their release from the megakaryocyte.35 To investigate the role of megakaryocytes in the production of TSP-1 during tumorigenesis, bone marrow megakaryocytes were isolated from the femurs of mice inoculated subcutaneously with Lewis lung carcinoma cells, after tumors reached 100 and 500 mm3. Western blot analyses revealed significant up-regulation of TSP-1 within the megakaryocytes isolated from femurs of tumor-bearing animals (Figure 4A). Quantitative polymerase chain reaction revealed a significant increase in the expression of Tsp-1 mRNA in tumor-bearing mice that correlated with increasing tumor volume (Figure 4B). To determine whether tumor growth affected megakaryocyte production, we isolated bone marrow cells from resting and tumor-bearing wild-type mice and immunostained these cells with anti-GPIBα antibody (a megakaryocyte-specific marker; Figure 4C). Quantification of GPIBα+ bone marrow cells demonstrated a significant increase in tumor-bearing mice compared with non–tumor-bearing animals (Figure 4C, P < .005). Similarly, hematoxylin and eosin staining of femur sections from both wild-type and Tsp-1−/− mice also demonstrated increased megakaryocyte lineage cells in the bone marrow of tumor-bearing animals based on size (Figure 4D). These data indicate that the increase in the total number of circulating platelets (supplemental Figure 4) may also be the result, in part, of an increase in megakaryopoiesis observed in tumor-bearing mice.
Megakaryopoiesis and TSP-1 expression are increased in the bone marrow of tumor-bearing wild-type mice. (A) Western blot for TSP-1 expression in megakaryocytes isolated from the bone marrow of Lewis lung tumor-bearing mice. β-Actin was probed as a loading control. (B) Tsp-1−/− mRNA levels in bone marrow megakaryocytes were quantified by quantitative polymerase chain reaction and compared with the housekeeping gene GAPDH before tumor growth (0), and when tumors reached 100 mm3 and 500 mm3 in volume. (C) Immunofluorescence analysis of bone marrow cytospins with anti-GPIBα (red) and Hoechst (blue) to detect megakaryocytes. Bar represents 50 μm. Right: Quantification of GPIBα+ cells in the bone marrow of non–tumor-bearing (0) or tumor-bearing mice (500 mm3). (D) Femurs from control and tumor-bearing wild-type and Tsp-1−/− mice were stained with hematoxylin and eosin. Arrows indicate megakaryocytes. Bar represents 50 μm.
Megakaryopoiesis and TSP-1 expression are increased in the bone marrow of tumor-bearing wild-type mice. (A) Western blot for TSP-1 expression in megakaryocytes isolated from the bone marrow of Lewis lung tumor-bearing mice. β-Actin was probed as a loading control. (B) Tsp-1−/− mRNA levels in bone marrow megakaryocytes were quantified by quantitative polymerase chain reaction and compared with the housekeeping gene GAPDH before tumor growth (0), and when tumors reached 100 mm3 and 500 mm3 in volume. (C) Immunofluorescence analysis of bone marrow cytospins with anti-GPIBα (red) and Hoechst (blue) to detect megakaryocytes. Bar represents 50 μm. Right: Quantification of GPIBα+ cells in the bone marrow of non–tumor-bearing (0) or tumor-bearing mice (500 mm3). (D) Femurs from control and tumor-bearing wild-type and Tsp-1−/− mice were stained with hematoxylin and eosin. Arrows indicate megakaryocytes. Bar represents 50 μm.
Platelets acquire TSP-1 through megakaryocytes
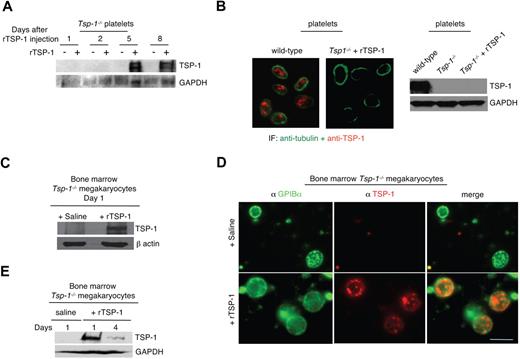
Anuclear platelets lack the machinery necessary for TSP-1 transcription. Thus, TSP-1 levels can increase in platelets of tumor-bearing mice through several possible mechanisms: direct uptake (endocytosis) by the circulating platelet, uptake (endocytosis) at the megakaryocyte level, and biosynthesis at the megakaryocyte level. To elucidate the mechanism by which platelets acquired additional TSP-1 and to determine whether rTSP-1 could be endocytosed and sequestered by platelets in vivo, we injected rTSP-1 via tail vein into Tsp-1−/− animals and isolated platelets at various times after injection. Western blot analysis of platelet lysates (Figure 5A) indicated that platelets did not directly take up rTSP-1 immediately after the injection. However, we did observe the appearance of TSP-1 in circulating platelets 5 days after rTSP-1 injection. Because it has been shown that platelet formation in mice requires 4.7 days before its release from the megakaryocytes,33,36 our result suggests that rTSP-1 uptake by platelets may occur via megakaryocyte uptake and packaging into assembling platelets. To rule out the possibility of direct uptake of TSP-1 by platelets, we coincubated Tsp-1−/− platelets and rTSP-1 ex vivo, followed by anti–TSP-1 immunofluorescence and Western blot analysis (Figure 5B). Our results suggest that platelets are unable to directly endocytose circulating rTSP-1 and therefore require the assistance of megakaryocytes.
Platelets acquire TSP-1 through megakaryocytes. (A) Western blot of TSP-1 expression in platelets harvested from Tsp-1−/− animals at indicated days after intravenous injection of 2 μg rTSP-1. GAPDH was probed as a loading control. (B) Left: Platelets from Tsp-1−/− mice are immunostained with antitubulin (green) and anti–TSP-1 (red) after coincubation at 37°C for 2 hours in vitro with 2 μg rTSP-1. Right: Western blot analysis of wild-type platelets, Tsp-1−/− platelets, and Tsp-1−/− platelets after in vitro coincubation with rTSP-1. GAPDH was probed as a loading control. (C) Western blot analysis of megakaryocytes isolated from the bone marrow of Tsp-1−/− mice after coincubation at 37°C for 2 hours in vitro with rTSP-1. β-Actin was probed as a loading control. (D) Immunostaining with anti-GPIBα (green) and anti–TSP-1 (red) of megakaryocytes isolated from the bone marrow of Tsp-1−/− mice 24 hours after intravenous injection of saline or 2 μg rTSP-1. Bar represents 50 μm. (E) Western blot analysis of megakaryocytes isolated from Tsp-1−/− mice 1 and 4 days after intravenous injection of 2 μg rTSP-1. GAPDH was probed as a loading control.
Platelets acquire TSP-1 through megakaryocytes. (A) Western blot of TSP-1 expression in platelets harvested from Tsp-1−/− animals at indicated days after intravenous injection of 2 μg rTSP-1. GAPDH was probed as a loading control. (B) Left: Platelets from Tsp-1−/− mice are immunostained with antitubulin (green) and anti–TSP-1 (red) after coincubation at 37°C for 2 hours in vitro with 2 μg rTSP-1. Right: Western blot analysis of wild-type platelets, Tsp-1−/− platelets, and Tsp-1−/− platelets after in vitro coincubation with rTSP-1. GAPDH was probed as a loading control. (C) Western blot analysis of megakaryocytes isolated from the bone marrow of Tsp-1−/− mice after coincubation at 37°C for 2 hours in vitro with rTSP-1. β-Actin was probed as a loading control. (D) Immunostaining with anti-GPIBα (green) and anti–TSP-1 (red) of megakaryocytes isolated from the bone marrow of Tsp-1−/− mice 24 hours after intravenous injection of saline or 2 μg rTSP-1. Bar represents 50 μm. (E) Western blot analysis of megakaryocytes isolated from Tsp-1−/− mice 1 and 4 days after intravenous injection of 2 μg rTSP-1. GAPDH was probed as a loading control.
We next assessed the role of megakaryocytes in the sequestration of rTSP-1. Bone marrow–derived Tsp-1−/− megakaryocytes were coincubated with rTSP-1 and analyzed by Western blot, demonstrating that megakaryocytes are able to directly endocytose rTSP-1 in vitro (Figure 5C). To examine whether megakaryocytes were able to take up rTSP-1 in vivo, we isolated femurs from Tsp-1−/− mice 24 hours after tail vein injection of rTSP-1. After megakaryocyte isolation, we costained cells with anti-GPIBα and anti–TSP-1 and observed TSP-1 expression within the megakaryocytes of mice injected with rTSP-1 versus the saline-injected control (Figure 5D). Western blot analysis of megakaryocytes isolated from the bone marrow further confirmed these results and demonstrated that rTSP-1 was detected within 1 day after rTSP-1 treatment and was still detectable up to 4 days after injection (Figure 5E).
Discussion
The role of endogenous angiogenesis inhibitors in suppressing the progression of in situ tumors and the significance of the balance between the angiogenic output of a tumor versus the host angiogenic defense have been previously described.2,37 Because platelets are able to scavenge tumor- and stroma-derived angiogenic factors, we wanted to determine whether tumor growth within an organism is reflected by the changes in the levels of the angiogenic regulators stored within platelets. We observed an increase in the levels of the potent endogenous angiogenesis inhibitor TSP-1 in platelets of tumor-bearing mice. This increase in TSP-1 directly correlated with tumor progression, and its expression in platelets resumed to baseline levels within 3 weeks after tumor resection. The observed inhibition of endothelial cell migration by platelet releasate from tumor-bearing animals shed some light on the possible functional significance of increases in platelet TSP-1. Because platelets are one of the largest single sources of angiogenic factors in vivo,38 we explored the role of platelets and specifically platelet-derived TSP-1 in tumor angiogenesis in vivo. Our results suggest that platelet-derived TSP-1 plays a role in the initial stages of tumor growth. Using transplantable tumor models, we demonstrated that the absence of TSP-1 from circulating platelets abrogated the ability of platelets to inhibit tumor angiogenesis in the earliest stages of tumor growth.
The relationship between platelets and malignancies has been studied for more than a century, with one of the earliest reports dating back to the 1800s.4 Elevated platelet counts are often associated with poor survival in a variety of cancers, including glioblastomas, gastric, colorectal cancers, and gynecologic malignancies. There is a strong body of evidence indicating a role for platelets in cancer metastasis, and treatments involving platelet-reducing agents have been beneficial in reducing the number of metastatic lesions in several mouse models.10,11,39,40 Despite platelets being one of the largest single sources of angiogenic regulators,38 their role in the earliest stages of tumor angiogenesis, regulation, and transport throughout the body has not been extensively studied. A single report described the involvement of platelets, and specifically TSP-1, in the regulation of angiogenesis during the reperfusion of ischemic limbs.24 This work demonstrated that TSP-deficient megakaryocytes and platelets manifest more robust proangiogenic activity than wild-type cells.24
TSP-1 is a potent endogenous angiogenesis inhibitor that normally makes up as much as 10% to 20% of the total platelet protein.16,41 There have been a limited number of studies investigating TSP-1 regulation; however, down-regulation of TSP-1 has been proposed as a common mechanism by which tumors increase neovascularization.42 TSP-1 expression has been shown to be down-regulated by loss of the p53 tumor suppressor gene19 or activation of the myc and ras oncogenes.43 Studies have also shown direct transcriptional up-regulation of Tsp-1 by p53 and transcriptional repression by c-Myc and Id-1.44-46 However, several other factors have been postulated to regulate TSP-1 expression, including changes in the expression of proangiogenic regulators within the tumor microenvironment, hormone regulation, and transforming growth factor-β, yet no molecular understanding exists of how any of these factors directly or indirectly regulates TSP-1. Under normal physiologic conditions, TSP-1 plasma levels are very low, whereas in cancer patients and certain mouse models, its levels have been shown to increase.47-51 One explanation for the observed increase in the plasma levels of TSP-1 in cancer patients could be its release from tumor-activated platelets, similar to the tumor-induced platelet release of VEGF, another angiogenic factor stored in platelets.52 In our study, neither serum nor plasma VEGF levels nor TSP-1 levels in tumor-bearing animals correlated with tumor volume (supplemental Figure 5; and data not shown), indicating that the observed TSP-1 plasma increase could be the result of nonspecific release by the platelets. Our results suggest that TSP-1 expression is critical for modulating the overall angiogenic function of platelets. We have previously demonstrated that proangiogenic and antiangiogenic factors are stored within separate platelet granules and that some of these proteins, such as VEGF and endostatin, are selectively released on platelet activation.5 Our observation that intraperitoneal injections of platelet contents are sufficient to alter tumor angiogenesis suggests the potent effect of local release of the angiogenic factors from platelets in regulating tumor angiogenesis. The rapid acceleration of tumor growth in wild-type mice bearing Tsp-1 null bone marrow compared with wild-type bone marrow offers strong evidence that the loss of TSP-1 from platelets shifts the microenvironment to a proangiogenic state favoring tumorigenesis. Furthermore, our results suggest that an increase in megakaryopoiesis and the subsequent increase in the numbers of circulating platelets with a concomitant increase in TSP-1 levels in platelets may be one of the earliest host responses to the growth of a tumor. It is fair to speculate that the actual increase in the expression of antiangiogenic TSP-1 and the platelet ability to deliver it to the site of the growing tumor may be sufficient to alter tumor progression by suppression of tumor angiogenesis. The observed delay in tumor growth of the Tsp-1−/− animals transplanted with wild-type bone marrow compared with Tsp-1−/− mice transplanted with Tsp-1−/− bone marrow indicates the significance of TSP-1 in the circulating platelets within tumor-bearing animals. Furthermore, the decrease in microvessel density in tumors harvested from mice reconstituted with Tsp-1−/− bone marrow supports the conclusion that platelet-derived TSP-1 is critical to suppress tumor angiogenesis.
Blood platelets arise through the formation of proplatelets by the terminally differentiated megakaryocytes within the bone marrow.35 Because platelets are anuclear, most proteins are either produced within the megakaryocytes or are acquired by platelets while in circulation. Our results indicate that, unlike VEGF (data not shown), platelets are not able to acquire TSP-1 directly from the circulation. The important role of megakaryocytes in packaging TSP-1 into circulating platelets suggests a tight and regulated control over platelet TSP-1. The significance of this observation remains to be further elucidated.
Interestingly, the observed up-regulation of TSP-1 and its subsequent increase in circulating platelets in tumor-bearing animals appear to be independent of tumor location. The reduction in platelet levels of TSP-1 after tumor resection suggests a possible future use of platelet TSP-1 expression as a candidate marker for monitoring tumor growth or recurrence. Our results further suggest that tumor-dependent megakaryopoiesis, an increase in the number of circulating platelets and TSP-1 expression, may be necessary to suppress tumor angiogenesis. Hence, in the clinical setting, TSP-1 may be a useful universal biomarker of tumor progression and recurrence. The ability to modulate levels of TSP-1 in the platelet α-granules and creation of “designer” platelets may have therapeutic potential in cancer. For instance, one could envision future treatments where, in addition to standard antiangiogenic and chemotherapy, cancer patients could also receive a transfusion of “designer” platelets, where the levels of antiangiogenic regulators significantly outweigh the levels of proangiogenic factors for therapy. Taken together, our data describe a novel mechanism of host suppression of tumorigenesis resulting from an increase in the number of circulating platelets and an increase in the megakaryocytic expression of TSP-1. Our studies indicate that platelet-derived TSP-1 plays a critical role in suppressing tumor angiogenesis in the earliest stages of tumor growth.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the members of the Italiano and Ryeom labs for helpful discussions and critical comments.
This study was supported by the National Institutes of Health (P01 CA045548-21; J.F., J.E.I., and S.R.).
National Institutes of Health
Authorship
Contribution: A.Z. designed and performed experiments, interpreted results, and wrote the manuscript; K.-H.B. and S.S. performed experiments, analyzed data, and interpreted results; R.C.L. performed experiments and interpreted results; J.G. performed experiments; J.F. designed experiments and interpreted results; J.E.I. designed experiments, interpreted results, formulated discussions, and assisted with manuscript preparation and editing; and S.R. designed experiments, interpreted results, formulated discussions, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sandra Ryeom, Department of Cancer Biology, Abramson Family Cancer Research Institute, University of Pennsylvania School of Medicine, 421 Curie Blvd, Philadelphia, PA 19104; e-mail: sryeom@upenn.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal