Abstract
The murine gut epithelium contains a large population of thymus-derived intraepithelial lymphocytes (IELs), including both conventional CD4+ and CD8αβ+ T cells (expressing T-cell receptor αβ [TCRαβ]) and unconventional CD8αα+ T cells (expressing either TCRαβ or TCRγδ). Whereas conventional IELs are widely accepted to arise from recirculation of activated CD4+ and CD8αβ+ T cells from the secondary lymphoid organs to the gut, the origin and developmental pathway of unconventional CD8αα IELs remain controversial. We show here that CD4-Cre-mediated inactivation of c-Myc, a broadly expressed transcription factor with a wide range of biologic activities, selectively impairs the development of CD8αα TCRαβ IELs. In the absence of c-Myc, CD4− CD8− TCRαβ+ thymic precursors of CD8αα TCRαβ IELs are present but fail to develop on adoptive transfer in immunoincompetent hosts. Residual c-Myc–deficient CD8αα TCRαβ IEL display reduced proliferation and increased apoptosis, which correlate with significantly decreased expression of interleukin-15 receptor subunits and lower levels of the antiapoptotic protein Bcl-2. Transgenic overexpression of human BCL-2 resulted in a pronounced rescue of CD8αα TCRαβ IEL in c-Myc–deficient mice. Taken together, our data support a model in which c-Myc controls the development of CD8αα TCRαβ IELs from thymic precursors by regulating interleukin-15 receptor expression and consequently Bcl-2–dependent survival.
Introduction
T lymphocytes can be broadly divided into 2 categories. Conventional T cells, expressing T-cell receptor αβ (TCRαβ) and either CD4 or CD8 coreceptors, arise in the thymus via a complicated differentiation process involving positive and negative selection and are subsequently exported to the periphery where they primarily reside in secondary lymphoid organs, such as lymph nodes and spleen. On the other hand, unconventional T cells (which can express either TCRαβ or TCRγδ with or without coreceptors) can be frequently found in nonlymphoid organs, such as the skin, liver, or intestine. Well-characterized examples of unconventional T cells in the mouse include dendritic epidermal T cells in the skin, Vα14 invariant (Vα14i) natural killer T (NKT) cells in the liver, and a subset of intraepithelial lymphocytes (IELs) expressing CD8αα homodimers in the gut. Unconventional T cells are generally accepted to be thymus-dependent and to undergo agonist selection during development, but details of their differentiation pathway and selection requirements are less well understood than for conventional T cells.1-4
Unconventional CD8αα IELs in the intestinal epithelium are composed of subsets expressing TCRαβ or TCRγδ. Despite early reports suggesting an extrathymic origin for these cells,5 more recent studies indicate that the majority of CD8αα TCRγδ T cells and (especially) CD8αα TCRαβ IELs are derived from thymic precursors that subsequently migrate to the gut.6,7 However, the differentiation pathway followed by CD8αα IELs remains controversial. According to one model, both CD8αα TCRγδ and CD8αα TCRαβ IELs originate from immature CD4−CD8− (DN) thymic precursors, which exit the thymus at the DN2-DN3 stage8 and complete their development in gut cryptopatches (CPs), which provide a unique microenvironment for the terminal differentiation of these cells.9 In contrast, another hypothesis proposes that CD8αα TCRαβ IELs ultimately arise from a unique CD8αα-expressing subset of CD4+ CD8αβ+ thymocytes (referred to as triple positive [TP]). According to this scenario, TP thymocytes undergo agonist selection to become DN TCRαβ+ cells, which subsequently leave the thymus and migrate to the gut to become CD8αα TCRαβ IELs under the local influence of interleukin-15 (IL-15).10 Although these conflicting studies appear difficult to reconcile (particularly with respect to the intrathymic differentiation pathway of CD8αα TCRαβ IELs), it should be noted that they are based on rather complex experimental protocols involving intrathymic injections and transplantations of thymocytes or whole thymi into immunodeficient recipients. Thus, there is a need for alternative approaches to investigate the origin and developmental pathway of CD8αα IELs.
Originally defined as an oncogene, c-Myc is a transcription factor that can play a role in proliferation, survival, apoptosis, or malignant transformation of diverse cell types depending on the tissue or context.11,12 In the thymus, early c-Myc inactivation in immature DN thymocytes leads to a severe block in T-cell development because of inhibition of pre-TCR–induced proliferation.13,14 In contrast, later inactivation of c-Myc in CD4+ CD8+ (DP) thymocytes has little effect on the subsequent development of conventional T cells. Interestingly, however, the development of several unconventional T-cell subsets, including Vα14i NKT cells and peripheral CD8 T cells with a memory phenotype (CD44high CD122+), is selectively impaired when c-Myc is inactivated at the DP thymocyte stage.15,16
Because CD8αα IELs share several properties with other unconventional T-cell subsets, such as Vα14i NKT cells and memory phenotype CD8 T cells (including an activated phenotype and dependence on IL-15 for development and/or survival), we investigated the role of c-Myc in CD8αα IEL development. Our data demonstrate that CD8αα TCRαβ (but not CD8αα TCRγδ) IELs are selectively decreased when c-Myc is inactivated at the DP thymocyte stage, probably because of a requirement for c-Myc in IL-15–dependent survival of these cells. The implications of these findings for current models of CD8αα IELs development will be discussed.
Methods
Mice
CD4-Cre c-mycflox/flox (c-mycΔCD4) mice have been previously described.16 CD4-Crenegc-mycflox/flox littermates were used as wild-type (WT) control mice. Transgenic mice expressing human BCL-2 under the control of the H2K promoter have been previously described.17 Rag2−/−γc−/− mice were purchased from Taconic Farms. c-mycN/N mice18 were kindly provided by Dr Frederick W. Alt (Boston, MA). IL15−/− mice19 were originally provided by Immunex. All mice are C57BL/6 background. Control C57BL/6 mice for the IL15−/− analysis were obtained from Harlan. Six- to 8-week-old mice were used in all experiments. All animal experiments were conducted under the authorization and with approval of the review board of the Veterinary Service from Canton de Vaud, Lausanne, Switzerland.
Cell preparations
Thymocyte and spleen cell suspensions were prepared by grinding the organs through mesh filters. CD8-depleted thymocytes were prepared as previously described.16 IELs were isolated as previously described.20 Briefly, Peyer patches were excised, and the small intestine was opened longitudinally and cut into 1-cm-long pieces. Then the specimens were washed twice in phosphate-buffered saline (PBS) containing 100 U/mL penicillin and 100 μg/mL streptomycin. The pieces were then stirred at 37°C in prewarmed Dulbecco modified Eagle medium containing 100 U/mL penicillin, 100 μg/mL streptomycin, and 5% fetal calf serum for 30 minutes. The supernatants were separated on a 40% to 70% Percoll density gradient (GE Healthcare). The cells that layered between the 40% and 70% fractions were collected as IELs.
Antibodies and flow cytometry
The following monoclonal antibody (mAb) conjugates were used: TCRγδ (GL3)-fluorescein isothiocyanate (FITC) and -phycoerythrin (PE); TCRβ (H57)-PE-Cy5.5, -Alexa647 and -allophycocyanin (APC)-Alexa 750; CD4 (GK1.5)-FITC, and -PE-Cy7; CD8α (53.6.7)-PE, -PE-Cy5.5, -PE-Cy7, -Alexa647, and -APC-Alexa 750; CD8β (H35)-PE and -Alexa 647; CD44 (IM781)-PE-Cy7 and -APC-Alexa 750; CD62L (Mel-14)-FITC; CD122 (IL-2Rβ, TM-β1)-PE and -biotin; CD132(γc chain, 4G3)-biotin; CD161 (PK136) -peridinin chlorophyll protein-Cy5.5; B220 (RA3.6B2)-PE-Cy7; IL-15Rα-biotin. All FITC conjugates were prepared in our laboratory using standard protocols. Alexa 647 and CD8α PE conjugates were also prepared in our laboratory using Alexa 647 protein labeling kit (Invitrogen) and the Prozyme PE conjugation kit (Europa Bioproducts), respectively. CD161 was obtained from BD Biosciences. IL-15Rα (BAF551) mAb was purchased from R&D Systems. CD122 and CD132 were purchased from BD Biosciences. The remaining mAbs were purchased from eBioscience. For revealing biotin-conjugates, PE-Streptavidin (eBioscience) was used.
CD8αα-expressing thymocytes were detected with PE-labeled TL-tetramer21 (kindly provided by Dr H. Cheroutre, La Jolla Institute for Allergy and Immunology, San Diego, CA), which was added before other mAbs used in the staining. TL-tetramer was detected with PE-Streptavidin from Invitrogen.
CD1d-hFc dimers for detecting Vα14i NKT cells have been previously described.22 Donkey anti–human-Fc-APC was purchased from Jackson ImmunoResearch Europe.
All cell suspensions were preincubated with 2.4G2 supernatant to block FcR, then washed and surface-stained using a combination of specific mAbs. For intracellular staining, cells were then fixed and permeabilized with BD Perm&Fix kit (BD Biosciences). Samples were analyzed on a FACSCanto flow cytometer (BD Biosciences). Data were analyzed using WinMDI software. Fluorescence-activated cell sorting was performed using a FACSAria Flow Cytometer (BD Biosciences).
Bcl-2 intracellular staining
Intracellular anti–mouse Bcl-2 (BD Biosciences) and anti–human BCL-2 (Dako Denmark) staining was performed according to the manufacturer's protocol. FITC-conjugated hamster IgG isotype control was used as control (BD Biosciences).
Cell-cycle and apoptosis analysis
Simultaneous cell-cycle analysis and 4-color surface staining were performed as previously described.23 Briefly, IELs were surface-labeled with the appropriate mAb conjugates, then fixed and permeabilized using BD Perm&Fix kit (BD Biosciences), and subsequently stained with anti-Ki67-FITC (B56; BD Biosciences) for 1 hour followed by Hoechst 33342 (Invitrogen) at 20 μg/mL for 5 minutes. After washing, cells were analyzed on a LSR2 Flow Cytometer (BD Biosciences). Doublets were eliminated using the DDM unit. For analysis of apoptosis, cells were stained with annexin V–Cy5 following the manufacturer's protocol (BD Biosciences).
In vitro cell culture
Electronically sorted DN TCRαβ+ B220− NK1.1− thymocytes from WT and c-mycΔCD4 mice were plated in 24-well plates (1 mL/105 cells/well) in complete RPMI 1640/10% fetal calf serum. A total of 100 ng/mL human IL-15 (hIL-15, R&D Systems) was added at day 0.
Adoptive transfer
Electronically sorted DN TCRαβ+ B220− NK1.1− thymocytes from WT and c-mycΔCD4 mice were intravenously transferred into Rag2−/−γc−/− mice (200 μL of PBS/2 × 105 cells). IELs were analyzed 4 weeks later.
Real-time PCR
Real-time polymerase chain reaction (PCR) using SYBR was performed on a LightCycler (Roche Diagnostic) according to the manufacturer's instructions. Total RNA from cell samples was purified using Trizol. Total RNA was reverse-transcribed using random nonamers and AMV reverse transcriptase (Roche Diagnostic). For the PCR, the LightCycler FastStart DNA Master SYBR Green I (Roche Diagnostic) was used according to the instruction manual. c-myc and N-myc transcripts were normalized to TATA-binding protein (TBP) as described elsewhere.24 Amplification plots were analyzed using the second derivative method with LightCycler data analysis software, Version 3.5 (Roche Diagnostic), and the relative quantification was determined using the LightCycler relative quantification software, Version 3.5 (Roche Diagnostic). Sextuplet analysis showed that measurement errors were always less than 9%.
Statistical analysis
Statistical analysis was performed using the Student t test.
Results
Selectively reduced numbers of CD8αα TCRαβ IELs in c-mycΔCD4 mice
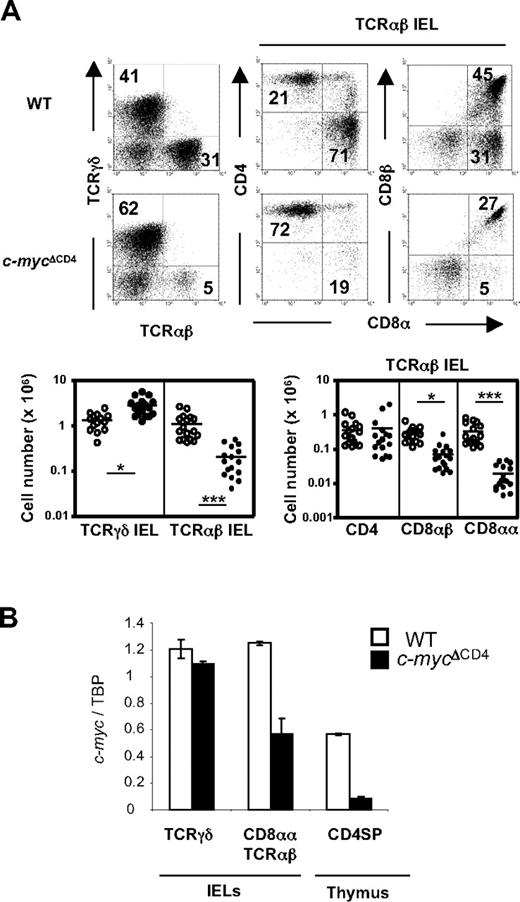
We have previously reported that inactivation of c-Myc at the DP stage of thymocyte development (in c-mycΔCD4 mice) does not affect further development of conventional CD4+ and CD8+ mature thymocytes or peripheral T cells. However, unconventional T-cell subsets, such as Vα14i NKT cells and memory phenotype (CD44high CD122+) CD8+ T cells, were selectively decreased in c-mycΔCD4 mice.16 Because the CD8αα TCRαβ subset of IEL shares several properties with these unconventional T cells (including an activated phenotype and dependence on IL-15 for development), we decided to investigate IEL development in c-mycΔCD4 mice. As shown in Figure 1A, TCRαβ IEL development was impaired in c-mycΔCD4 mice, whereas TCRγδ IELs were present in normal (or even slightly increased) numbers. Further analysis of TCRαβ IELs in c-mycΔCD4 mice revealed that the unconventional CD8αα subset was dramatically (∼ 20-fold) reduced, whereas the CD8αβ subset was only slightly (∼ 3-fold) decreased and the CD4 subset was normal (Figure 1A). These data thus indicate a critical and selective role for c-Myc in CD8αα TCRαβ IEL development. Interestingly, quantitative PCR analysis of c-myc transcripts in residual CD8αα TCRαβ IELs in c-mycΔCD4 mice revealed only a 2-fold decrease compared with WT controls (Figure 1B), suggesting that these may represent rare cells that have survived by escaping c-myc deletion on one allele.
Selective impairment of CD8αα TCRαβ IEL development in c-mycΔCD4 mice. (A) IELs from WT and c-mycΔCD4 mice were stained with TCRγδ, TCRαβ, CD4, CD8α, and CD8β. Dot plots represent staining of TCRγδ versus TCRαβ in total IELs, and CD4 versus CD8α and CD8β versus CD8α in the TCRαβ+ IEL population. Numbers in the dot plots indicate the percentage of cells represented in the quadrant. The charts below the dot plots represent the absolute numbers of the indicated IEL subsets in individual mice (○ represents WT mice; and ●, c-mycΔCD4 mice). Horizontal line in each group represents the mean value. Statistically significant differences: *P < .05, ***P < .001. (B) cDNA from the indicated sorted populations was assayed for c-myc expression by quantitative PCR. c-myc levels were normalized to TBP and presented in arbitrary units. Error bars indicate mean ± SD.
Selective impairment of CD8αα TCRαβ IEL development in c-mycΔCD4 mice. (A) IELs from WT and c-mycΔCD4 mice were stained with TCRγδ, TCRαβ, CD4, CD8α, and CD8β. Dot plots represent staining of TCRγδ versus TCRαβ in total IELs, and CD4 versus CD8α and CD8β versus CD8α in the TCRαβ+ IEL population. Numbers in the dot plots indicate the percentage of cells represented in the quadrant. The charts below the dot plots represent the absolute numbers of the indicated IEL subsets in individual mice (○ represents WT mice; and ●, c-mycΔCD4 mice). Horizontal line in each group represents the mean value. Statistically significant differences: *P < .05, ***P < .001. (B) cDNA from the indicated sorted populations was assayed for c-myc expression by quantitative PCR. c-myc levels were normalized to TBP and presented in arbitrary units. Error bars indicate mean ± SD.
Normal numbers of putative thymic precursors of CD8αα TCRαβ IEL in c-mycΔCD4 mice
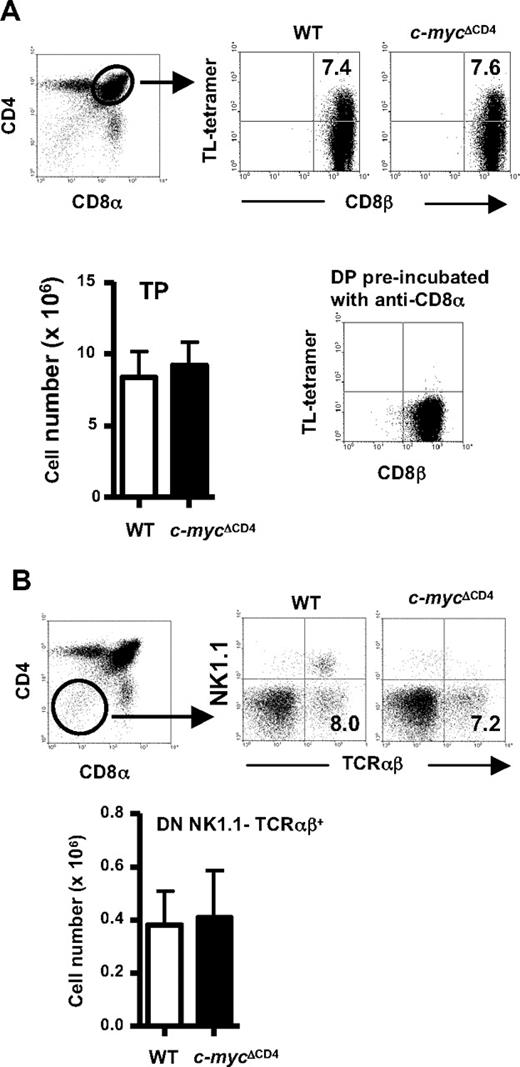
Recent evidence suggests that CD8αα TCRαβ IELs are derived from thymic precursors by a process involving agonist selection. According to this model, TP thymocytes expressing CD8αα homodimers (which can be detected by staining with TL tetramers) are the preselection precursors of CD8αα TCRαβ IELs. After agonist selection, TP thymocytes differentiate to become DN NK1.1− TCRαβ+ (DN TCRαβ+) thymocytes, which can give rise to CD8αα TCRαβ IELs when transferred into RAG2−/− mice.10 We therefore investigated whether either of these putative IEL precursor populations was affected in c-mycΔCD4 mice. As shown in Figure 2, both TP thymocytes (Figure 2A) and DN TCRαβ+ thymocytes (Figure 2B) were present in normal frequencies and absolute numbers in c-mycΔCD4 mice.
Normal numbers of putative thymic CD8αα TCRαβ IEL precursors in c-mycΔCD4 mice. A representative CD4 versus CD8α thymocyte profile (identical in WT and c-mycΔCD4 mice) is shown to indicate the gates used for panels A and B. (A) Analysis of putative thymic preselection IEL precursors in WT and c-mycΔCD4 mice. Total thymocytes were stained with CD4, CD8α, CD8β, and TL-tetramer. CD4+ CD8α+ thymocytes were analyzed for coexpression of TL-tetramer and CD8β. Numbers in the dot plots indicate percentages. A control experiment for the TL-tetramer staining was performed by incubating thymocytes first with anti-CD8α and subsequently with TL-tetramer. Bar graph represents absolute numbers (mean ± SD; n = 6) of CD4+ CD8αβ+ TL-tetramer+ (TP) thymocytes. (B) Analysis of putative thymic postselection IEL precursors in WT and c-mycΔCD4 mice. Total thymocytes were stained with CD4, CD8α, B220, NK1.1, and TCRαβ. DN B220− thymocytes were analyzed for NK1.1 and TCRαβ expression. Numbers in the quadrants indicate percentages. Bar graph represents absolute numbers (mean ± SD; n = 6) of DN NK1.1− B220− TCRαβ+ thymocytes.
Normal numbers of putative thymic CD8αα TCRαβ IEL precursors in c-mycΔCD4 mice. A representative CD4 versus CD8α thymocyte profile (identical in WT and c-mycΔCD4 mice) is shown to indicate the gates used for panels A and B. (A) Analysis of putative thymic preselection IEL precursors in WT and c-mycΔCD4 mice. Total thymocytes were stained with CD4, CD8α, CD8β, and TL-tetramer. CD4+ CD8α+ thymocytes were analyzed for coexpression of TL-tetramer and CD8β. Numbers in the dot plots indicate percentages. A control experiment for the TL-tetramer staining was performed by incubating thymocytes first with anti-CD8α and subsequently with TL-tetramer. Bar graph represents absolute numbers (mean ± SD; n = 6) of CD4+ CD8αβ+ TL-tetramer+ (TP) thymocytes. (B) Analysis of putative thymic postselection IEL precursors in WT and c-mycΔCD4 mice. Total thymocytes were stained with CD4, CD8α, B220, NK1.1, and TCRαβ. DN B220− thymocytes were analyzed for NK1.1 and TCRαβ expression. Numbers in the quadrants indicate percentages. Bar graph represents absolute numbers (mean ± SD; n = 6) of DN NK1.1− B220− TCRαβ+ thymocytes.
Impaired IL-15–dependent differentiation of DN TCRαβ+ thymic precursors of CD8αα TCRαβ IELs in c-mycΔCD4 mice
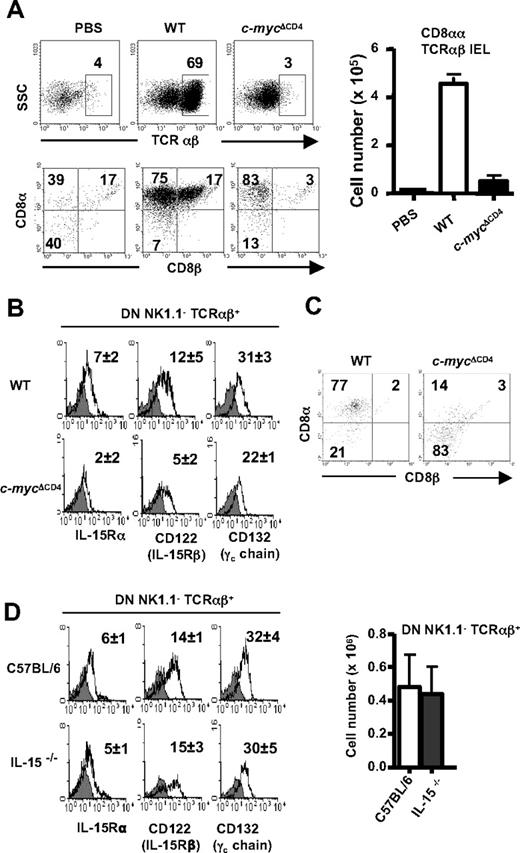
The final maturation of CD8αα TCRαβ IELs, including the induction of CD8αα expression, occurs only after thymus export in the IL-15–rich environment of the gut. Because intrathymic precursor numbers appeared normal in c-mycΔCD4 mice, we therefore asked whether c-Myc affects postthymic CD8αα TCRαβ IEL differentiation. To this end, we transferred sorted DN TCRαβ+ thymocytes from WT or c-mycΔCD4 mice to Rag2−/−γc−/− mice and analyzed the IEL subsets of recipients 1 month later. In contrast to WT, transferred cells from c-mycΔCD4 mice could not give rise to CD8αα TCRαβ IELs (Figure 3A). Because IL-15 is important for the final maturation of CD8αα TCRαβ IELs in the gut,25,26 we examined the expression of IL-15Rα, β, and γ chains on DN TCRαβ+ thymocytes in c-mycΔCD4 mice. Compared with control mice, the expression of IL-15Rα, β, and γ chains was decreased significantly in DN TCRαβ+ thymocytes from c-mycΔCD4 mice (Figure 3B), which might result in reduced sensitivity of these cells to IL-15 signaling. To test this hypothesis directly, we cultured sorted DN TCRαβ+ thymocytes from WT or c-mycΔCD4 mice with recombinant IL-15 for 6 days. The results showed that DN TCRαβ+ thymocytes from WT mice can give rise to CD8αα progeny in the presence of IL-15, whereas DN TCRαβ+ thymocytes from c-mycΔCD4 mice cannot (Figure 3C). Taken together, these results suggest that the lower expression of IL-15R components on DN TCRαβ+ thymocytes from c-mycΔCD4 mice might be responsible for the impaired postthymic maturation of CD8αα TCRαβ IEL.
Role of c-Myc in IL-15–dependent differentiation of DN TCRαβ+ precursors of CD8αα TCRαβ IELs. (A) IEL reconstitution on transfer of electronically sorted DN B220− NK1.1− TCRαβ+ thymocytes from WT and c-mycΔCD4 mice into Rag2−γc− recipient mice. PBS alone was injected as negative control. Mice were analyzed 1 month after the transfer. TCRαβ IELs (identified in top panels) were stained with CD8α versus CD8β (bottom panels). Bar graph represents absolute numbers (mean ± SD; n = 5) of CD8αα TCRαβ IELs. (B) Histograms represent surface expression of IL-15R chains on DN NK1.1−B220− TCRαβ+ thymocytes from WT and c-mycΔCD4 mice. The number in each histogram corresponds to the mean fluorescence intensity (MFI) of the IL-15R staining (empty histogram) minus the MFI of the unstained control (gray filled histogram) (mean ± SD; n = 5). (C) DN B220− NK1.1− TCRαβ+ thymocytes from WT and c-mycΔCD4 were electronically sorted and cultured with 100 ng/mL hIL-15 for 6 days. After culture, cells were counted (the absolute number of viable cells was similar for WT and c-mycΔCD4 mice) and stained with TCRαβ, CD8α, and CD8β. Dot plots show CD8α versus CD8β profile of TCRαβ+ cells. Numbers in the dot plots indicate percentages. Data are representative of 2 independent experiments. (D) Histograms show surface expression of IL-15R chains on DN NK1.1−B220− TCRαβ+ thymocytes from control C57BL/6 and IL15−/− mice. The number in each histogram corresponds to the MFI of the IL-15R staining (empty histogram) minus the MFI of the unstained control (gray filled histogram) (mean ± SD; n ≥ 4). Bar graph represents absolute number of DN NK1.1− TCRαβ+ thymocytes in control C57BL/6 and IL-15−/− mice (mean ± SD; n ≥ 4).
Role of c-Myc in IL-15–dependent differentiation of DN TCRαβ+ precursors of CD8αα TCRαβ IELs. (A) IEL reconstitution on transfer of electronically sorted DN B220− NK1.1− TCRαβ+ thymocytes from WT and c-mycΔCD4 mice into Rag2−γc− recipient mice. PBS alone was injected as negative control. Mice were analyzed 1 month after the transfer. TCRαβ IELs (identified in top panels) were stained with CD8α versus CD8β (bottom panels). Bar graph represents absolute numbers (mean ± SD; n = 5) of CD8αα TCRαβ IELs. (B) Histograms represent surface expression of IL-15R chains on DN NK1.1−B220− TCRαβ+ thymocytes from WT and c-mycΔCD4 mice. The number in each histogram corresponds to the mean fluorescence intensity (MFI) of the IL-15R staining (empty histogram) minus the MFI of the unstained control (gray filled histogram) (mean ± SD; n = 5). (C) DN B220− NK1.1− TCRαβ+ thymocytes from WT and c-mycΔCD4 were electronically sorted and cultured with 100 ng/mL hIL-15 for 6 days. After culture, cells were counted (the absolute number of viable cells was similar for WT and c-mycΔCD4 mice) and stained with TCRαβ, CD8α, and CD8β. Dot plots show CD8α versus CD8β profile of TCRαβ+ cells. Numbers in the dot plots indicate percentages. Data are representative of 2 independent experiments. (D) Histograms show surface expression of IL-15R chains on DN NK1.1−B220− TCRαβ+ thymocytes from control C57BL/6 and IL15−/− mice. The number in each histogram corresponds to the MFI of the IL-15R staining (empty histogram) minus the MFI of the unstained control (gray filled histogram) (mean ± SD; n ≥ 4). Bar graph represents absolute number of DN NK1.1− TCRαβ+ thymocytes in control C57BL/6 and IL-15−/− mice (mean ± SD; n ≥ 4).
c-Myc acts upstream of IL-15 signaling in DN TCRαβ+ thymocytes
The reduced expression of IL-15R components and impaired IL-15 responsiveness in vitro of c-Myc–deficient DN TCRαβ+ thymocytes suggest that c-Myc may be acting upstream of IL-15 signaling to inhibit postthymic maturation of these cells into CD8αα TCRαβ IELs. Alternatively, it remains theoretically possible that c-Myc acts downstream of IL-15 signaling during CD8αα TCRαβ IEL development, as suggested previously for memory CD8 T-cell development.27 To accommodate this latter scenario, one must hypothesize that interference with downstream IL-15 signaling in the absence of c-Myc would initiate a homeostatic feedback loop that ultimately results in down-regulation of expression of IL-15R components on DN TCRαβ+ thymocytes. To distinguish between these possibilities, we examined IL-15R expression in DN TCRαβ+ thymocytes from IL-15−/− mice, which have impaired CD8αα TCRαβ IEL development because of lack of IL-15 signaling19 but no defect in c-Myc. As shown in Figure 3D, DN TCRαβ+ thymocytes were present in normal numbers in IL-15−/− mice and expressed similar levels of IL-15Rα, β, and γ chains as control mice, despite the absence of IL-15 signaling. These data exclude an indirect role for c-Myc in regulating IL-15R expression via a putative homeostatic IL-15–dependent feedback loop and thus support the more straightforward hypothesis that c-Myc acts upstream of IL-15R components in DN TCRαβ+ thymic precursors to regulate IL-15 signaling during CD8αα TCRαβ IEL development.
c-Myc affects IL-15R expression, proliferation, and survival of CD8αα TCRαβ IELs
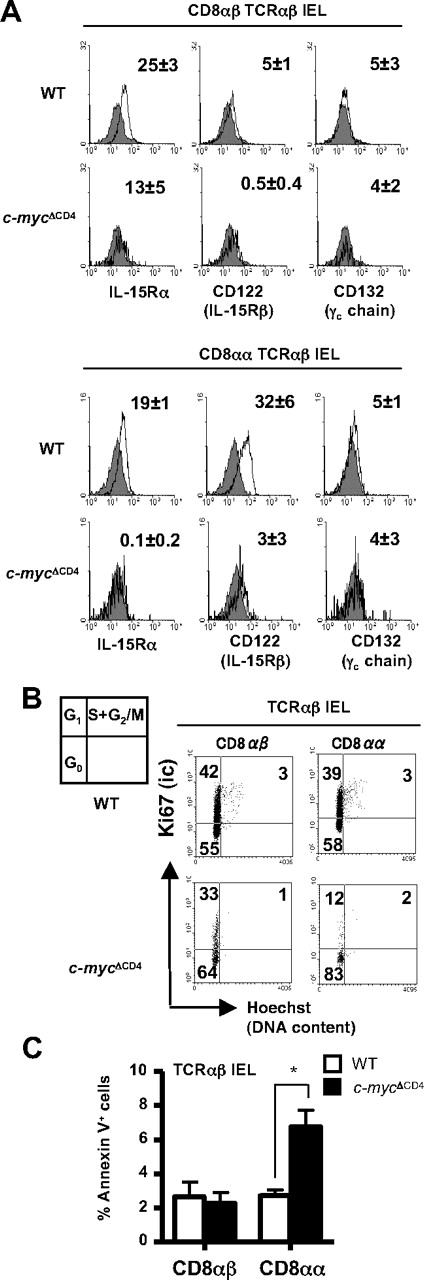
Because IL-15 has been reported to be a major survival factor for NK cells, NKT cells, and IELs,19 we investigated whether c-Myc plays a role in the IL-15–dependent survival of IELs. Importantly, the expression of IL-15Rα and IL-15Rβ chains on CD8+ TCRαβ IELs, especially on CD8αα TCRαβ IELs, was much lower in c-mycΔCD4 mice compared with control mice (Figure 4A). Consistent with this, CD8αα TCRαβ IELs in c-mycΔCD4 mice showed poorer proliferation and higher apoptosis (Figure 4B-C). These results suggest that c-Myc affects postthymic differentiation of CD8αα TCRαβ IELs by regulating IL-15–dependent proliferation and/or survival.
c-Myc deficiency affects IL-15R expression, proliferation, and survival of CD8αα TCRαβ IELs. (A) Expression of IL-15R chains on CD8αα and CD8αβ TCRαβ IEL subsets from WT and c-mycΔCD4 mice. The number in each histogram corresponds to MFI of the IL-15R staining (empty histogram) minus MFI of the unstained control (gray filled histogram) (mean ± SD; n = 4). (B) Cell-cycle analysis of TCRαβ IELs in WT and c-mycΔCD4 mice. Dot plots show Ki67 versus Hoechst profile for CD8αβ and CD8αα TCRαβ IELs. Numbers in dot plots correspond to percentages of cells in G0, G1, and S + G2/M. Results are representative of 2 independent analyses. (C) Bar graph represents the percentage of apoptotic (annexin V+ 7-amino-actinomycin D−) CD8αβ and CD8αα TCRαβ IELs in WT and c-mycΔCD4 mice (mean ± SD; n = 6). *Statistically significant difference (P < .05).
c-Myc deficiency affects IL-15R expression, proliferation, and survival of CD8αα TCRαβ IELs. (A) Expression of IL-15R chains on CD8αα and CD8αβ TCRαβ IEL subsets from WT and c-mycΔCD4 mice. The number in each histogram corresponds to MFI of the IL-15R staining (empty histogram) minus MFI of the unstained control (gray filled histogram) (mean ± SD; n = 4). (B) Cell-cycle analysis of TCRαβ IELs in WT and c-mycΔCD4 mice. Dot plots show Ki67 versus Hoechst profile for CD8αβ and CD8αα TCRαβ IELs. Numbers in dot plots correspond to percentages of cells in G0, G1, and S + G2/M. Results are representative of 2 independent analyses. (C) Bar graph represents the percentage of apoptotic (annexin V+ 7-amino-actinomycin D−) CD8αβ and CD8αα TCRαβ IELs in WT and c-mycΔCD4 mice (mean ± SD; n = 6). *Statistically significant difference (P < .05).
CD8αα TCRαβ IELs in c-mycΔCD4 mice are partially rescued by enforced BCL-2 expression
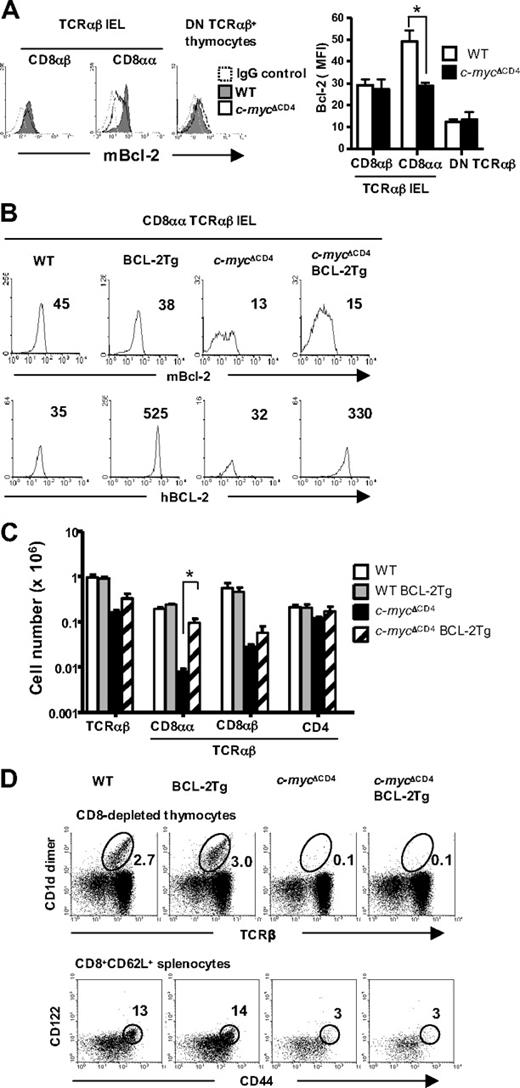
Because CD8αα TCRαβ IELs in c-mycΔCD4 mice preferentially underwent apoptosis, we examined the expression of the antiapoptotic protein Bcl-2 in these cells. Indeed, Bcl-2 levels were significantly reduced in CD8αα TCRαβ IELs from c-mycΔCD4 mice but remained normal in CD8αβ TCRαβ IELs as well as in DN TCRαβ+ thymic precursors (Figure 5A). To directly examine the role of Bcl-2 in IEL development, we introduced a human BCL-2 transgene driven by an H2K promoter17 into c-mycΔCD4 mice. Expression of exogenous human BCL-2 in CD8αα TCRαβ IELs was comparable in both BCL-2 Tg and c-mycΔCD4 BCL-2 Tg mice, and expression levels of endogenous mouse Bcl-2 were not affected by the presence of the transgene (Figure 5B). Importantly, the enforced expression of BCL-2 selectively restored the numbers of CD8αα TCRαβ IELs in c-mycΔCD4 mice (10-fold increase) without rescuing the CD8αβ TCRαβ IEL subset (Figure 5C).
CD8αα TCRαβ IEL development in c-mycΔCD4 mice is partially restored by enforced BCL-2 expression. (A) Intracellular mouse Bcl-2 expression in the indicated populations from WT and c-mycΔCD4 mice. Dashed histogram represents the staining with IgG control. Bar graph represents MFI of mouse Bcl-2 staining (mean ± SD; n = 6). *P < .05. (B) Intracellular mouse Bcl-2 or human BCL-2 expression in CD8αα TCRαβ IELs from WT, BCL-2 Tg, c-mycΔCD4, and c-mycΔCD4 BCL-2 Tg mice. The number in each histogram indicates the MFI of Bcl-2 staining. (C) Bar graph represents the absolute number of cells in the indicated IEL subsets in WT, BCL-2 Tg, c-mycΔCD4, and c-mycΔCD4 BCL-2 Tg mice (mean ± SD; n = 5). *Statistically significant difference (P < .05). (D) Thymic Vα14i NKT cells and memory phenotype CD8 splenic T cells in WT, BCL-2 Tg, c-mycΔCD4, and c-mycΔCD4 BCL-2 Tg mice. CD8-depleted thymocytes were stained with TCRβ and CD1d-dimer. Percentage of Vα14i NKT cells (TCRβ+ CD1d-dimer+) is indicated in the upper dot plots. Total spleen cell suspensions were stained with CD8α, CD62L, CD122, and CD44. Lower dot plots represent CD122 versus CD44 profile of the CD8α+ CD62L+ splenic T cells. The percentage of memory phenotype T cells (CD122+ CD44+) is indicated. Data are representative of 3 independent experiments.
CD8αα TCRαβ IEL development in c-mycΔCD4 mice is partially restored by enforced BCL-2 expression. (A) Intracellular mouse Bcl-2 expression in the indicated populations from WT and c-mycΔCD4 mice. Dashed histogram represents the staining with IgG control. Bar graph represents MFI of mouse Bcl-2 staining (mean ± SD; n = 6). *P < .05. (B) Intracellular mouse Bcl-2 or human BCL-2 expression in CD8αα TCRαβ IELs from WT, BCL-2 Tg, c-mycΔCD4, and c-mycΔCD4 BCL-2 Tg mice. The number in each histogram indicates the MFI of Bcl-2 staining. (C) Bar graph represents the absolute number of cells in the indicated IEL subsets in WT, BCL-2 Tg, c-mycΔCD4, and c-mycΔCD4 BCL-2 Tg mice (mean ± SD; n = 5). *Statistically significant difference (P < .05). (D) Thymic Vα14i NKT cells and memory phenotype CD8 splenic T cells in WT, BCL-2 Tg, c-mycΔCD4, and c-mycΔCD4 BCL-2 Tg mice. CD8-depleted thymocytes were stained with TCRβ and CD1d-dimer. Percentage of Vα14i NKT cells (TCRβ+ CD1d-dimer+) is indicated in the upper dot plots. Total spleen cell suspensions were stained with CD8α, CD62L, CD122, and CD44. Lower dot plots represent CD122 versus CD44 profile of the CD8α+ CD62L+ splenic T cells. The percentage of memory phenotype T cells (CD122+ CD44+) is indicated. Data are representative of 3 independent experiments.
The rescue of CD8αα TCRαβ IELs by BCL-2 in c-mycΔCD4 was not the result of restoration of IL-15R expression because levels of all IL-15R chains remained very low on CD8αα TCRαβ IELs and DN TCRαβ+ thymocytes in c-mycΔCD4 BCL-2 Tg mice (data not shown). Moreover, the promotion of survival by BCL-2 in c-mycΔCD4 mice was restricted to the CD8αα TCRαβ IEL subset because no rescue of Vα14i NKT cells or memory phenotype (CD44high CD122+) CD8+ splenic T cells was observed (Figure 5D).
N-myc expressed from the c-myc locus can rescue CD8αα TCRαβ IEL development
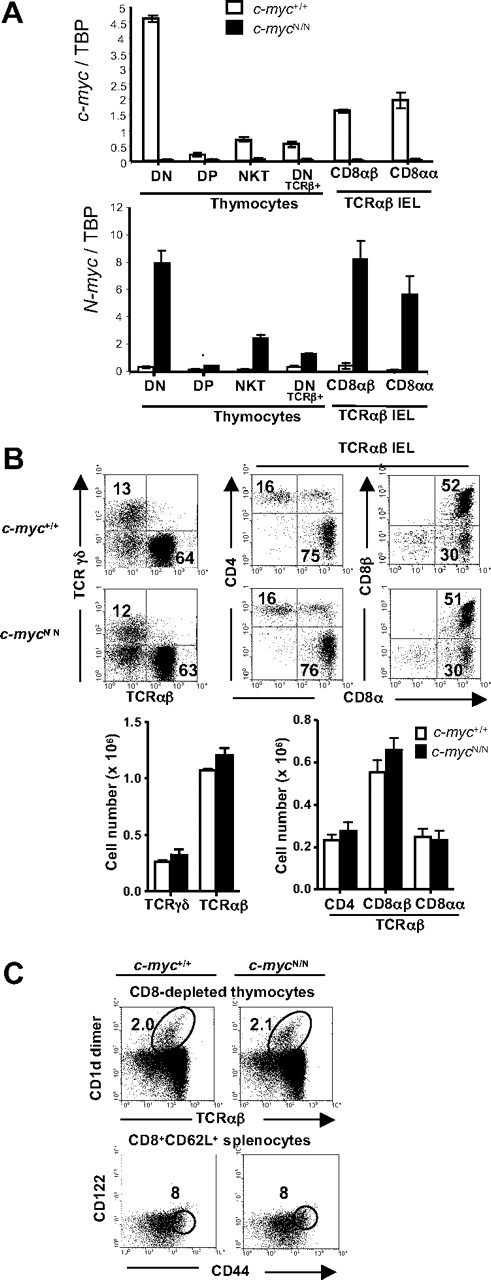
The failure of other Myc family members to rescue CD8αα TCRαβ IEL development (as well as the development of Vα14i NKT cells and CD44high CD122+ CD8+ T cells) in c-mycΔCD4 mice could be interpreted to mean that c-Myc has a unique function during the development of unconventional T-cell subsets. However, both N-Myc and L-Myc are expressed at extremely low levels in DP thymocytes and mature T cells,16 which could quantitatively preclude their ability to replace c-Myc in the T-cell lineage. To address this issue directly, we took advantage of the availability of c-mycN/N knock-in mice, which express N-Myc coding sequences from the c-myc locus on both alleles, whereas c-Myc expression is completely disrupted. Previous studies have shown that c-mycN/N mice are viable and that development of conventional T and B cells proceeds normally in these mice.18 As shown in Figure 6A, c-mycN/N mice are devoid of detectable c-myc transcripts in thymocytes as well as in Vα14i NKT cells and IELs. Moreover, N-myc transcripts are dramatically increased in c-mycN/N mice and exhibit the same pattern of expression as c-myc transcripts in normal mice (c-myc+/+, Figure 6A). Importantly, analysis of IELs in c-mycN/N mice and littermate controls demonstrates normal percentages and absolute numbers of all subsets, including CD8αα TCRαβ IELs (Figure 6B). In addition, Vα14i NKT cells and CD44high CD122+ CD8+ splenic T cells were present at the same levels in c-mycN/N mice compared with littermate controls (Figure 6C). Together with previous studies,18 these data demonstrate that c-Myc and N-Myc are potentially functionally redundant during unconventional and conventional T-cell development, provided that they are expressed under the control of the same genetic regulatory elements.
Normal development of unconventional T cells in c-mycN/N mice. (A) cDNA from the indicated populations from control (white bars) and c-mycN/N (black bars) mice was assayed for c-myc and N-myc expression by quantitative RT-PCR. Myc levels were normalized to TBP and are presented in arbitrary units. (B) Flow cytometric analysis of IELs from control and c-mycN/N mice. Samples were stained and analyzed as in Figure 1. Numbers indicate the percentage of cells in each quadrant. Bar graphs represent absolute numbers (mean ± SD; n = 5) of the indicated IEL subsets. (C) Thymic Vα14i NKT cells and splenic memory phenotype CD8+ T cells in WT and c-mycN/N mice. Samples were stained and analyzed as in Figure 5D. Data are representative of 2 independent experiments.
Normal development of unconventional T cells in c-mycN/N mice. (A) cDNA from the indicated populations from control (white bars) and c-mycN/N (black bars) mice was assayed for c-myc and N-myc expression by quantitative RT-PCR. Myc levels were normalized to TBP and are presented in arbitrary units. (B) Flow cytometric analysis of IELs from control and c-mycN/N mice. Samples were stained and analyzed as in Figure 1. Numbers indicate the percentage of cells in each quadrant. Bar graphs represent absolute numbers (mean ± SD; n = 5) of the indicated IEL subsets. (C) Thymic Vα14i NKT cells and splenic memory phenotype CD8+ T cells in WT and c-mycN/N mice. Samples were stained and analyzed as in Figure 5D. Data are representative of 2 independent experiments.
Discussion
The data presented here demonstrate that c-Myc plays a critical and selective role in the development of CD8αα TCRαβ IELs. Inactivation of a floxed c-myc allele in DP thymocytes via a CD4-Cre transgene had little effect on the further development of conventional CD4+ and CD8+ mature thymocytes and peripheral T cells. However CD8+ TCRαβ IELs were significantly reduced, largely resulting from a 20-fold reduction in absolute numbers of the CD8αα TCRαβ subset. Moreover, residual CD8αα TCRαβ IELs in c-mycΔCD4 mice displayed reduced proliferation and increased apoptosis, which correlated with decreased expression of IL-15R subunits and significantly lower levels of expression of the antiapoptotic protein Bcl-2. Importantly, transgenic overexpression of human BCL-2 led to a pronounced (∼ 10-fold) increase in the absolute numbers of CD8αα TCRαβ IELs in c-mycΔCD4 mice. Taken together with other studies,25,26 our results support a model in which c-Myc regulates the survival of CD8αα TCRαβ IELs in the intestine by controlling IL-15R expression, which in turn regulates levels of intracellular Bcl-2.
In addition to identifying a selective role for c-Myc in CD8αα TCRαβ IEL development, our data have interesting implications in light of the current controversy concerning the origin of this unusual cell population. Although CD8αα TCRαβ IELs are generally considered to be ultimately of thymic origin (at least in euthymic mice), the precise identification of intrathymic precursors of CD8αα TCRαβ IELs and their subsequent intrathymic and extrathymic differentiation pathway are intensely debated subjects. According to one school of thought, CD8αα TCRαβ IELs derive from early intrathymic precursors (DN2–3) that exit the thymus and colonize CP in the gut epithelium, where they further differentiate to become mature CD8αα TCRαβ IELs.7,8 Another model posits that CD8αα TCRαβ IELs are derived from CD8αα DP thymocyte precursors (referred to as TP) that are induced by agonist selection to develop into DN TCRαβ+ intermediates, which subsequently leave the thymus and home to the gut where they mature into CD8αα TCRαβ IELs under the local influence of IL-15.6,10
In our model system, c-myc inactivation by the CD4-Cre transgene should occur relatively late in thymus development (beyond the DN3 stage) and hence would not be expected to impair CD8αα TCRαβ IEL development if these cells were exclusively (or mainly) derived from early thymic progenitors (DN2–3). Indeed, premature expression of the CD4-Cre transgene in DN2/3 thymocytes of c-mycΔCD4 mice can be formally excluded because deletion of c-Myc at this stage (using a Lck-Cre transgene) has been shown to severely impair subsequent thymocyte development.13 Based on these considerations, we favor the hypothesis that the impaired development of CD8αα TCRαβ IELs in c-mycΔCD4 mice reflects an essential role for c-Myc during their intrathymic and/or extrathymic development at or beyond the DP thymic progenitor stage. In this regard, our data are compatible with genetic fate-mapping experiments28 showing that CD8αα TCRαβ IELs were marked by a green fluorescent protein reporter after CD4-Cre activation, whereas CD4+ lymphoid inducer cells present in CP were not. These latter experiments were likewise interpreted to mean that CD8αα TCRαβ IELs are the progeny of DP thymocytes.28
Another aspect of our study relevant to the controversy surrounding the developmental origin of CD8αα TCRαβ IELs is the observation that c-Myc–deficient DN TCRαβ+ thymocytes were unable to develop into CD8αα TCRαβ IELs after intravenous transfer in Rag2−/−γc−/− mice, whereas the WT DN TCRαβ+ subset generated CD8αα TCRαβ IELs under these conditions as previously described.10 The failure of DN TCRαβ+ thymocytes from c-mycΔCD4 mice to generate CD8αα TCRαβ IELs on intravenous transfer is most probably related to defective IL-15 signaling because these cells expressed lower levels of IL-15R subunits and were impaired in their ability to give rise to CD8αα TCRαβ cells when cultured in vitro with recombinant IL-15. Although our data do not directly address the controversial issue of whether DN TCRαβ+ thymocytes are derived from TP thymocytes, they do provide strong support in favor of the hypothesis that DN TCRαβ+ thymocytes are developmental intermediates in CD8αα TCRαβ IEL differentiation. Moreover, reduced IL-15R expression in DN TCRαβ+ thymocytes from c-mycΔCD4 mice is consistent with the possibility that these cells have undergone impaired intrathymic agonist selection in the absence of c-Myc.
The identification of c-Myc as a critical regulator of CD8αα TCRαβ IEL development confirms and extends a more general emerging role for c-Myc in unconventional T-cell lineages. Thus, in addition to CD8αα TCRαβ IELs, c-Myc is essential for the development of Vα14i NKT cells and memory phenotype CD44high CD122+ CD8+ peripheral T cells.15,16,27 Interestingly, all of these unconventional T-cell subsets dependent on c-Myc share several properties, including a constitutively activated phenotype and a requirement for IL-15 for their development and/or homeostasis.29 Although the precise molecular mechanisms controlling the selective c-Myc requirement in unconventional T-cell development remain to be elucidated, our present study reveals subset-specific effects of introducing a human BCL-2 transgene in c-mycΔCD4 mice. Thus, CD8αα TCRαβ IEL development is largely restored (10-fold increase in absolute numbers) by exogenous BCL-2 expression, whereas Vα14i NKT and CD44high CD122+ CD8+ T cells are unaffected. The reason for this discrepancy is not clear but could potentially reflect a preferential effect of c-Myc deficiency on survival in the CD8αα TCRαβ IEL lineage (which could be rescued by BCL-2) compared with proliferation15 or other functions in Vα14i NKT cells and memory CD44high CD122+ CD8+ peripheral T cells (which would not be rescued by BCL-2). Analysis of the role of c-Myc in the development of other IL-15–dependent cell subsets, such as CD8αα NK1.1+ T cells,30 might help to clarify this issue.
Finally, our data are also of interest with regard to the potential functional redundancy of the Myc protein family. Of the 3 family members (c-Myc, N-Myc, and L-Myc), only c-Myc is expressed at significant levels in the T-cell lineage from the DP thymocyte stage onward,16 perhaps explaining why c-Myc deficiency in unconventional T cells cannot be rescued by N-Myc or L-Myc. Direct evidence in favor of Myc functional redundancy was obtained using knock-in mice that express N-myc from both alleles of the c-myc locus. Despite the total absence of c-Myc in these mice, not only CD8αα TCRαβ IELs but also Vα14i NKT cells and CD44high CD122+ CD8+ peripheral T cells were present at comparable levels as in WT littermate controls. Thus, adequate levels of N-Myc are sufficient to substitute for c-Myc in the development of 3 independent lineages of unconventional T cells. In an earlier study, conventional T-cell development was found to be normal in c-mycN/N mice, but some subtle defects in T-cell (and B-cell) activation were observed.18 Further studies are thus required to unambiguously determine whether Myc family members function in a “generic” fashion or exhibit unique tissue-specific activities.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr H. Cheroutre for TL tetramers, Dr D. Littman for the CD4-Cre mice, Dr F. W. Alt for the c-mycN/N mice, Dr J. Domen for the H2K-BCL-2 transgenic mice, Marcin Mycko for initial breeding of c-mycΔCD4 mice, Anne Wilson for help in cell-cycle analysis, Catherine Fumey for technical assistance, and Danny Labes for fluorescence-activated cell sorting.
This work was supported in part by the Swiss National Science Foundation (3100A0–120165; H.R.M.), the Swiss Cancer League (OCS-01863–02-2006; A.T.), and the BMBF program HaematoSys.
Authorship
Contribution: W.J. designed, performed, and analyzed the majority of the experiments of this work; I.F. designed, performed, and analyzed the experiments of real-time PCR and contributed to other experiments; E.L. carried out the breeding of c-mycN/N mice and contributed to the analysis of these mice; A.T. provided critical mice for this study; H.R.M. directed the study, planned experiments, and wrote the manuscript; and W.J. and I.F. helped with the writing and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: H. Robson MacDonald, Ludwig Institute for Cancer Research, Lausanne Branch, University of Lausanne, CH-1066 Epalinges, Switzerland; e-mail: hughrobson.macdonald@licr.unil.ch.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal