Certain chemotherapeutics are now known to augment host immunity by acting on DCs. In this issue of Blood, Nakahara and colleagues demonstrate a unique pharmacologic activity of CTX to selectively eliminate the lymphoid tissue-resident CD8+ DC subset in mice.1
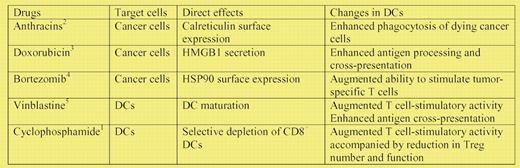
Although chemotherapeutic agents are generally believed to suppress the host immunity, recent studies have unveiled unexpected potentials of some agents to augment the adaptive immune responses against cancer cells (see table). For example, anthracins (eg, doxorubicin and mitoxantrone), but not other DNA-damaging drugs, induce an immunogenic form of cancer cell death characterized by surface expression of calreticulin, which in turn promotes efficient phagocytosis of dying cancer cells by dendritic cells (DCs).2 Cancer cells being killed by doxorubicin also secrete the high-mobility-group box 1 (HMGB1), which augments antigen processing and presentation by DCs in a TLR4-dependent manner.3 Similarly, bortezomib, an inhibitor of 26S proteasome, induces surface expression of heat shock protein 90 (HSP90) on cancer cells, thereby inducing DC maturation indirectly.4 Through unbiased screening of a relatively large number of chemotherapeutic drugs, vinblastin was found to directly trigger phenotypic and functional maturation of DCs.5 Although cyclophosphamide (CTX) is reported in the literature to potentiate adaptive immune responses against established tumors, at least in part by abrogating regulatory T cells, (Tregs),6,7 the underlying mechanisms responsible still remain unclear.
In the current study, Nakahara et al tested the impact of single administration of CTX on 3 distinct DC subsets in skin-draining lymph nodes in mice: (1) DCs derived from Langerhans cells and dermal DCs, termed the migratory DCs; (2) lymphoid tissue-resident CD8+ DCs; and (3) plasmacytoid DCs (pDCs).1 Although the numbers of all 3 DC subsets were significantly reduced by CTX, the CD8+ resident DC subset was almost completely depleted within 4 days after CTX treatment. All DC subsets eventually returned to the baseline levels. The resulting transient imbalance in DC subsets, in turn, led to enhancement of the T cell–stimulatory function of the DC populations as a whole, suggesting the immuno-inhibitory potential of the CD8+ DC subset. In the in vitro antigen presentation assays, crude DC preparations isolated from CTX-injected mice induced marked expansion of a Treg population with an unusual property of interferon-γ (IFNγ) production. This effect of CTX treatment was reversed by adding the purified CD8+ DC population back to the assays. Moreover, those IFNγ-producing Tregs that had been expanded with DCs from CTX-injected mice exhibited functional impairment in their in vitro ability to suppress the growth of conventional T cells.
In vivo relevance of these findings was elegantly demonstrated in mice using implantable tumor models. When adoptively transferred into tumor-bearing mice, CD8 T cells expressing transgenic T-cell receptors for a specific tumor-associated antigen exhibited clonal expansion and IFNγ production. Importantly, CTX administration further augmented such CD8 T cell responses, and this in vivo effect of CTX was abrogated by intradermal injection of CD8+, but not CD8−, DC preparations. In a different set of experiments, CTX administration was found to promote the rejection of tumors upon second tumor challenge, and this effect was also diminished significantly by injection of CD8+ DCs, but not CD8− DCs. Thus, it appears reasonable to conclude that selective and transient depletion of the CD8+ DC subset accounts for the augmented immune status observed after CTX treatment.
It is now evident that different DC subsets play distinct functional roles. When activated with pathogenic stimuli, CD8+ resident DCs are fully capable of capturing and presenting various forms of exogenous antigens. In the steady state, however, CD8+ DCs serve as regulatory DCs maintaining the peripheral tolerance via preferential activation of Tregs.8 Thus, selective elimination of the CD8+ resident DC population may produce opposing immunologic outcomes depending on the microenvironment in the lymphoid tissues. In the absence of pathogenic signals, CTX-induced depletion of immature CD8+ DCs may augment host immune responses against cancer cells, as has been described here. On the other hand, patients receiving CTX treatments may fail to mount protective immunity against infectious pathogens due to the deficiency in mature CD8+ DCs.
The study by Nakahara et al was not designed to elucidate mechanisms by which CTX impairs the balance among the tested DC subsets. CTX may have killed the CD8+ resident DC subset selectively. Alternatively, CTX may have induced preferential expansion of the migratory DC and pDC subsets. In this regard, Salem et al reported that bone marrow cells isolated from CTX-treated mice produced higher numbers of DCs in culture9 and that CTX administration augmented in situ proliferation of DCs in the bone marrow during the early phase and then in the blood and spleen during the recovery phase.10 Likewise, working with a hepatic tumor metastasis model, Radojcic et al found that CTX administration elevated the number of tumor-infiltrating DCs in mice by promoting the rebound expansion of DC progenitors.11 The observed imbalance may have been simply caused by the difference in turnover among the tested DC subsets—CD8+ resident DCs are regarded as one of the DC subsets showing the most rapid rate of turnover.8 Obviously, further studies are required to determine the underlying mechanisms. Nevertheless, the current study provides a new conceptual basis for the use of CTX as an “immuno-stimulatory” chemotherapeutic drug.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■
REFERENCES
National Institutes of Health

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal