Although AKT is essential for multiple cellular functions, the role of this kinase family in hematopoietic stem cells (HSCs) is unknown. Thus, we analyzed HSC function in mice deficient in the 2 isoforms most highly expressed in the hematopoietic compartment, AKT1 and AKT2. Although loss of either isoform had only a minimal effect on HSC function, AKT1/2 double-deficient HSCs competed poorly against wild-type cells in the development of myeloid and lymphoid cells in in vivo reconstitution assays. Serial transplantations revealed an essential role for AKT1 and AKT2 in the maintenance of long-term HSCs (LT-HSCs). AKT1/2 double-deficient LT-HSCs were found to persist in the G0 phase of the cell cycle, suggesting that the long-term functional defects are caused by increased quiescence. Furthermore, we found that the intracellular content of reactive oxygen species (ROS) is dependent on AKT because double-deficient HSCs demonstrate decreased ROS. The importance of maintaining ROS for HSC differentiation was shown by a rescue of the differentiation defect after pharmacologically increasing ROS levels in double-deficient HSCs. These data implicate AKT1 and AKT2 as critical regulators of LT-HSC function and suggest that defective ROS homeostasis may contribute to failed hematopoiesis.
Introduction
The hematopoietic system is in a constant state of self-renewal as stem cells continuously replenish short-lived blood cells.1 All blood cells are derived from the long-term hematopoietic stem cell (LT-HSC) subset that maintains peripheral homeostasis by undergoing continual self-renewal for the life of the organism. LT-HSCs differentiate into multipotent progenitor cells (MPPs), a more mature subset that lacks the long-term ability to self-renew but retains the capacity to reconstitute all blood lineages.1,2 Both LT-HSCs and MPPs are found in the lineage-negative, c-Kit–positive, and Sca-1–positive (LSK) populations.3 Studying the LSK subset itself has contributed to the understanding of HSC biology because it is a population highly enriched for HSCs. Within the LSK subset, those cells positive for CD150 expression and negative for CD48 identify LT-HSCs that are capable of long-term self-renewal (CD150+CD48−LSK), whereas those cells that are negative for both (CD150−CD48−LSK) identify a multipotential population that has a comparably limited contribution to long-term hematopoiesis and is enriched for MPPs.1,4,5 Although definitive HSC experiments require a test of the functional capacity of the populations, the surface phenotype of these populations allows for an estimation of the presence of stem cells with long-term self-renewal capacity or those with more limited hematopoietic potential.
Among the signal transduction pathways that have attracted considerable attention as possibly being involved in HSC self-renewal is the phosphoinositide 3-kinase (PI3K)-AKT pathway. PI3K is a lipid kinase6 critical for the activation of AKT, a family of serine threonine kinases essential for the control of cellular metabolism and survival in multiple tissues.7,8 Haneline et al9 demonstrated that HSCs with decreased PI3K activity exhibit defective hematopoietic reconstitution and a reduced proliferative capacity. Concordantly, conditional deletion of phosphatase and tensin homolog (PTEN), a phosphatase that negatively regulates PI3K,10 promotes differentiation and proliferation at the expense of self-renewal, leading to depletion of the HSC pool.11,12 Researchers have also examined the importance of molecules downstream of the PI3K/AKT pathway. For instance, FOXO, a family of transcription factors negatively regulated by AKT,13 controls HSC quiescence by maintaining a low threshold of intracellular reactive oxygen species (ROS).14 HSCs that lack multiple FOXO family members are hyperproliferative and fail to self-renew but are normalized by treatment with antioxidants.14
A recent report by Kharas et al15 showed that constitutive activation of AKT in hematopoietic HSCs results in a hyperproliferative state and subsequent HSC depletion, akin to the phenotype of PTEN-deleted HSCs. However, to fully appreciate the biologic role of AKT in HSC development, complementary studies in the absence of AKT are necessary. A main challenge to depleting AKT from HSCs is the expression of 3 isoforms in mammalian cells: AKT1, AKT2, and AKT3. AKT1 and AKT2 are ubiquitously expressed and in greater abundance in hematopoietic cells,16,,–19 whereas AKT3 expression is most pronounced in the testes and brain but also can be expressed in lesser amounts in the hematopoietic system.20 Mogi et al21 showed that HSCs from mice deficient in only AKT1 or AKT2 are functionally normal, but a more complete analysis of the role of AKT isoforms in HSC function has been made difficult by the fact that mice lacking both AKT1 and AKT2 die in the early postnatal period.22 However, indirect evidence suggests that AKT may play an important role in HSC function. It has been reported that AKT becomes phosphorylated and presumably activated during the LT-HSC to MPP differentiation stage,23 through the action of stem cell factor, a cytokine required for HSC function,24,25 among others. In general, hematopoietic subsets that are simultaneously undergoing differentiation and proliferation may require sufficient AKT signaling to maintain survival.19
To address more fully the possible role(s) played by AKT1 and AKT2 in HSCs, we combined both in vitro and in vivo methods to study murine HSCs deficient in either AKT1, AKT2, or both. We found that HSCs are minimally affected by the loss of either isoform, but the combined absence of AKT1 and AKT2 impairs the long-term self-renewal capacity of HSCs. Our data also support the model that AKT activation is required to maintain proliferation in CD150+CD48− LSKs and survival in CD150−CD48−LSKs. Furthermore, we found that AKT regulates intracellular ROS content in HSCs and that a sufficient level of ROS is necessary for HSC function.
Methods
Mice
Mice lacking AKT1 or AKT2 on the C57BL/6 background were described previously.17,18 B6.Ly5SJL mice were purchased from The Jackson Laboratory. All experiments were performed according to the guidelines of the University of Pennsylvania Institutional Animal Care and Use Committee with the committee's ethical approval.
Flow cytometric analysis
Single-cell suspensions from thymi, spleens, and red blood cell–lysed bone marrow were stained in FACS buffer (Hanks balanced salt solution [HBSS], 2% fetal bovine serum) and analyzed on a FACSCalibur or an LSRII (BD Biosciences), and raw data were analyzed with FlowJo software (Version 8.8; TreeStar Inc). Annexin V was measured by use of the manufacturer's protocol (BD Biosciences) except for an additional wash step before analysis. 4′,6-diamidino-2-phenylindole (DAPI) was used as a live/dead exclusion marker for all LSRII and FACSAria experiments. All flow cytometry used an LSRII (BD Biosciences), with the exceptions of the ROS assay (FACSCalibur) and cell sorting (FACSAria). The lineage cocktail in bone marrow consisted of NK1.1 (PK136), CD11b (M1/70), Gr-1 (8C5), CD11c (HL3), T-cell receptorγδ (TCRγδ; GL3), B220 (RA3-6B2), Ter119, TCRβ (H57-597), CD8 (53-6.7), CD4 (RM4-5), CD3 (2C11), and CD19 (6D5; all BD Biosciences). The lineage cocktail for the fetal liver (FL) cells was modified by excluding CD11b. Other antibodies used included CD117/cKit (2B8), Sca-1 (E13-161.7), CD45.1/Ly5SJL (A20), CD45.2/Ly5B6 (104), CD34 (RAM34), and FcγRII/III (2.4G2) from BD Biosciences and CD150 (TC15-12F12.2) and CD48 (HM48-1) from BioLegend. All flow cytometric samples were treated with FcBlock (2.4G2; BD Biosciences) before incubation with primary antibodies except when identifying the bone marrow myeloid subsets. The total cellularity was derived by multiplying the total trypan blue–negative cells with the frequency of cells found in the indicated populations contained within the DAPI-negative and FSC/SSC gates.
Measurement of ROS
Freshly sorted LSK cells were incubated with 2μM CM-H2DCFDA (Molecular Probes) resuspended in warm HBSS or HBSS alone for unstained controls for exactly 15 minutes. The cells were immediately analyzed on a FACSCalibur, and the MFI was compared with unstained control cells. Thymocytes from bone marrow chimeras were surface stained with CD4, CD8, CD25, and CD45.2 in HBSS on ice, pelleted, and then resuspended in 2μM CM-H2DCFDA (DCF-DA; Molecular Probes).
Statistical analysis
Statistical analysis was performed with the use of 2-tailed, unpaired Student t test with Prism software version 5.0a (GraphPad Inc). Significance is denoted with asterisks (ie, *P < .05, **P < .01, ***P < .001).
Real-time polymerase chain reaction
Total RNA was isolated with TRIzol Reagent (Invitrogen). RNA was used to prepare cDNA with First Strand cDNA synthesis (GE Healthcare). All samples were normalized to GAPDH RNA transcript levels. Murine Akt3 primers and probes were purchased from Applied Biosystems. Samples were run on a 7900HT Sequence Detection System (Applied Biosystems) and analyzed with the use of SDS 2.1 (Applied Biosystems).
Bone marrow chimeras
FLs were harvested from E14.5 to E16.5 embryos and cultured overnight at 4°C in α-minimum essential medium supplemented with 20% fetal bovine serum, 2.2 g/L sodium bicarbonate, 2mM glutamine, penicillin, streptomycin, 10 ng/mL interleukin-6 (IL-6; Peprotech), 20 ng/mL IL-3 (Peprotech), and 100 μg/mL stem cell factor (Peprotech).26 Embryos were genotyped by polymerase chain reaction amplification, and 0.25 × 106 cells were injected into irradiated B6.Ly5SJL mice. Recipient mice were given 2 radiation doses of 550 rad, 4 hours apart. Donor cells were injected retrooribitally 4 hours after the last irradiation. Serially transplanted mice were injected with 2 to 5 × 106 freshly isolated red blood cell–lysed bone marrow cells mixed from 2 to 3 donors. Mice were maintained on antibiotic water (400 mg of sulfamethoxazole and 80 mg of trimethoprim per 500 mL of sterile water) for more than 4 weeks after reconstitution.
FL harvest
Compound mutant AKT1+/−AKT2+/− breeding pairs (Ly5B6+) or congenic (CD45.1+ B6.Ly5SJL) control breeding pairs were monitored for plugs and harvested 14.5 to 16.5 days after conception. FLs were red blood cell lysed and placed into media (see “Bone marrow chimeras”) overnight at 4°C. Individual fetuses were genotyped, and 2 fetuses with the same genotype were mixed to control for mouse-to-mouse variation. Littermates were used to control for background variation.
Hoechst/pyronin Y
Freshly isolated bone marrow cells (1 × 107 cells/mL) were incubated with 10 μg/mL Hoechst 33342 (Molecular Probes) diluted in Dulbecco modified Eagle medium for 45 minutes at 37°C. Then, the cells were stained with surface antibodies for 30 minutes at 4°C, washed once, and fixed with 1 mL of 5% paraformaldehyde (Electron Microscopy Sciences). After an overnight incubation in 5% paraformaldehyde at 4°C, Pyronin Y (Polysciences Inc) was added to a final concentration of 1 μg/mL, incubated for 30 minutes at 4°C, and immediately analyzed on the LSRII (BD Biosciences). Verapamil (Sigma-Aldrich) was added at a concentration of 50 μg/mL to all solutions before paraformaldehyde fixation.
Methylcellulose assay
Whole FL cells or sorted LSK cells from bone marrow chimeras were plated in duplicate in MethoCult M3434 (StemCell Technologies Inc) and cultured at 37°C in 5% CO2 for 10 days before counting. Colony morphology was scored on the basis of StemCell Technologies criteria, but no differences were detected in the number of colony subtypes that grew from mutant versus wild type in all experiments. L-Butathionine-sulfoximine (BSO; Sigma-Aldrich) was added to methylcellulose media at the indicated concentrations at the time of plating.
Long-term culture-initiating cell assay
Whole FL cells or sorted LSK cells were plated on an irradiated feeder layer of OP9-GFP cells27 and cultured in long-term culture-initiating cell (LTC-IC) media plus dexamethasone (StemCell Technologies Inc) at 32°C in 5% CO2 with the use of 24-well plates. Half of the media was discarded, and fresh media was added weekly according to the protocol from StemCell Technologies. After 2 weeks, the wells were harvested and counted, then equal numbers of cells were plated onto methylcellulose cultures in duplicate (MethoCult M3434; StemCell Technologies Inc). The diversity of cultures was noted, but statistically significant differences were not found. Fresh BSO (Sigma-Aldrich) was added to the LTC-IC media at the indicated concentrations on day 0 and with each weekly media change.
Peripheral blood analysis
A 50-μL heparin-treated whole blood aliquot per animal was diluted with 200 μL of 5% bovine serum albumin in phosphate-buffered saline and analyzed with an ADVIA 2120 Hematology System (Bayer Diagnostic) calibrated daily in the clinical hematology laboratory at Children's Hospital of Philadelphia.
Results
Single deficiency of AKT1 or AKT2 has a minimal effect on hematopoiesis
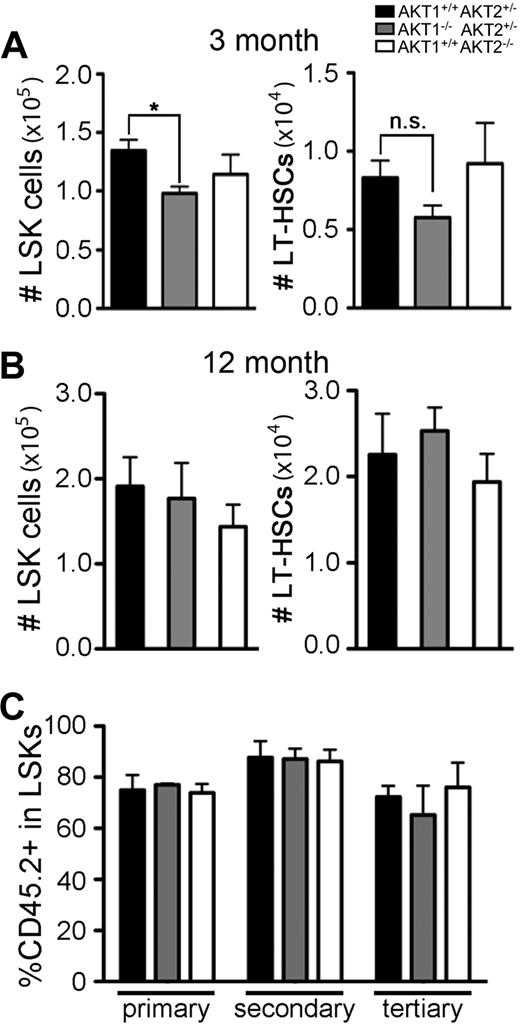
To begin to address the role of AKT in HSC function, global hematopoietic development in the absence of AKT1 or AKT2 was assessed. Overall cellularity and distribution of mature myeloid and lymphoid subsets in peripheral lymphoid organs were not affected by the loss of AKT1 or AKT2 in adult mice (supplemental Figure 1A-B, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Young adult AKT1−/− animals had reduced numbers of LSK cells (Figure 1A left) as well as modest reductions in LT-HSCs (CD150+CD48−LSK; Figure 1A right). However, LSK cellularity had normalized by the time these animals reached 12 months of age (Figure 1B left). The difference in LSK cellularity may be caused by the smaller body size of AKT1−/− mice compared with littermate controls.16,18 However, measurements of the frequency of LSKs and LT-HSCs also showed decreased frequencies of these populations in total bone marrow in AKT1−/− 3-month-old but not 12-month-old mice (data not shown). Single deficiency of AKT2 had no effect on LSK or LT-HSC cellularity in either 3-month-old or 12-month-old mice (Figure 1A-B).
Quantification of AKT1−/− or AKT2−/− HSCs in adult mice and assessment of function in serial transplantation. Total number of LSKs and LT-HSCs (CD150+CD48−LSKs) in bone marrow from femurs and tibias in (A) 3-month-old or (B) 12-month-old single knockout mice. Graphs represent the mean ± SEM in (A) n = 6 and (B) n = 4. (C) Percentage of single knockout (CD45.2+Ly5B6+) cells in the bone marrow LSK population 16 weeks after the initial transplantation (primary) and then 16 weeks after each serial transplantation (secondary and tertiary). FL cells from single-knockout fetuses (CD45.2+Ly5B6+) were mixed with wild-type FL cells (CD45.1+ B6.Ly5SJL) in a 3:1 (CD45.2+:CD45.1+) ratio and serially transplanted every 16 weeks. Bar graphs represent the mean ± SEM (n = 4).
Quantification of AKT1−/− or AKT2−/− HSCs in adult mice and assessment of function in serial transplantation. Total number of LSKs and LT-HSCs (CD150+CD48−LSKs) in bone marrow from femurs and tibias in (A) 3-month-old or (B) 12-month-old single knockout mice. Graphs represent the mean ± SEM in (A) n = 6 and (B) n = 4. (C) Percentage of single knockout (CD45.2+Ly5B6+) cells in the bone marrow LSK population 16 weeks after the initial transplantation (primary) and then 16 weeks after each serial transplantation (secondary and tertiary). FL cells from single-knockout fetuses (CD45.2+Ly5B6+) were mixed with wild-type FL cells (CD45.1+ B6.Ly5SJL) in a 3:1 (CD45.2+:CD45.1+) ratio and serially transplanted every 16 weeks. Bar graphs represent the mean ± SEM (n = 4).
We next tested the ability of singly deficient HSCs to self-renew in a stringent assay for stem cell function.28,29 FL cells lacking either AKT1, AKT2, or littermate controls (allotypically marked with CD45.2+Ly5B6+) were mixed in a 3:1 ratio with congenic (CD45.1+B6.Ly5SJL) wild-type FL cells, injected into irradiated CD45.1+ B6.Ly5SJL hosts (primary transplantation), and then subjected to secondary and tertiary serial transplantations. Neither loss of AKT1 nor AKT2 affected LSK reconstitution after 48 weeks in serial transplantations (Figure 1C). However, mature lymphoid cells but not myeloid cells failed to develop in the periphery (supplemental Figure 1C). Collectively, these data show that neither AKT1 nor AKT2 alone is absolutely required for HSC self-renewal, suggesting that if there is a requirement for AKT, these 2 isoforms may compensate for each other.
Short-term hematopoiesis is mildly affected by the combined loss of AKT1 and AKT2
Individual loss of AKT1 or AKT2 was shown previously to have only a minor effect on αβT-cell development, whereas the loss of both isoforms results in a profound defect.19,30,31 To test whether HSCs demonstrate a similar pattern of functional overlap, HSC function was tested by the use of cells that lacked both AKT1 and AKT2. Because AKT1−/−AKT2−/− mice are perinatal lethal, all subsequent experiments were performed with FL-derived HSCs (FL-HSCs). Furthermore, we measured Akt3 mRNA expression, in the event that the absence of both AKT1 and AKT2 would increase expression of the remaining isoform, AKT3, relative to AKT1- and AKT2-sufficient cells. However, we found that Akt3 expression was unchanged in Akt1−/−Akt2−/− LSK cells compared with controls (supplemental Figure 2).
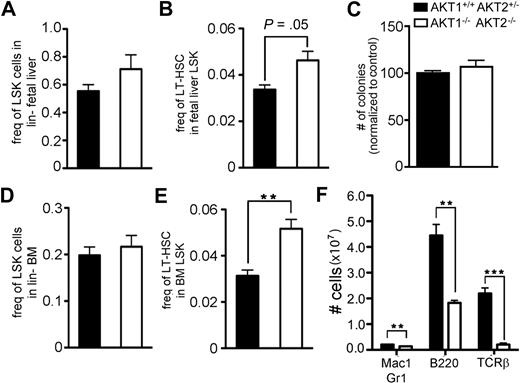
AKT1−/−AKT2−/− fetuses are approximately 50% smaller than littermate controls, making absolute comparisons of total fetal HSCs difficult to interpret.22 However, the frequency of all LSK cells was enriched in AKT1−/−AKT2−/− fetuses compared with their littermate controls (Figure 2A). In addition, this result was statistically significant when analyzing the frequency of LT-HSCs (CD150+CD48−LSK cells) in AKT1−/−AKT2−/− FLs relative to littermate controls (Figure 2B). This relative augmentation of LT-HSCs in AKT1−/−AKT2−/− fetuses may be the result of (1) an accumulation of LT-HSCs that fail to differentiate, (2) a loss of more mature LSK cells but isolated preservation of the LT-HSC subset, or (3) a combination of failure to differentiate from LT-HSCs and failed maintenance of mature LSK cells.
Central and peripheral hematopoietic reconstitution by AKT1−/−AKT2−/− FL-derived HSCs. The frequency of (A) LSK cells in the lineage-negative gate or (B) LT-HSCs (CD150+CD48−LSKs) in the total LSK gate of E14.5 FLs. Graphs represent the mean ± SEM (n = 5). (C) The total number of colonies generated 10 days after equal numbers of FL cells were plated in methylcellulose media. The diversity of colonies was not different between the 2 genotypes. Data are represented as the percentage of wild-type colonies generated within each experiment and represent the mean ± SEM (n = 4). The frequency of (D) LSK cells in the lineage-negative gate or (E) LT-HSCs (CD150+CD48−LSKs) in the total LSK gate of bone marrow from irradiated mice reconstituted 12 weeks previously with FL cells of the indicated genotype. Graphs represent the mean ± SEM (n = 3). (F) The total number of cells in the splenic myeloid (Mac-1+Gr-1+), B (B220+), and T (TCRβ+) lineages in mice from panels D and E. Graphs represent the mean ± SEM (n = 3).
Central and peripheral hematopoietic reconstitution by AKT1−/−AKT2−/− FL-derived HSCs. The frequency of (A) LSK cells in the lineage-negative gate or (B) LT-HSCs (CD150+CD48−LSKs) in the total LSK gate of E14.5 FLs. Graphs represent the mean ± SEM (n = 5). (C) The total number of colonies generated 10 days after equal numbers of FL cells were plated in methylcellulose media. The diversity of colonies was not different between the 2 genotypes. Data are represented as the percentage of wild-type colonies generated within each experiment and represent the mean ± SEM (n = 4). The frequency of (D) LSK cells in the lineage-negative gate or (E) LT-HSCs (CD150+CD48−LSKs) in the total LSK gate of bone marrow from irradiated mice reconstituted 12 weeks previously with FL cells of the indicated genotype. Graphs represent the mean ± SEM (n = 3). (F) The total number of cells in the splenic myeloid (Mac-1+Gr-1+), B (B220+), and T (TCRβ+) lineages in mice from panels D and E. Graphs represent the mean ± SEM (n = 3).
To test the differentiation capacity of progenitor cells, we compared their colony-forming capacity in semisolid media. We found that loss of both AKT1 and AKT2 allowed for differentiation to the myeloid lineage because cells from the double knockout animal formed similar numbers and distribution of colony subsets in vitro (Figure 2C and data not shown). HSC self-renewal and differentiation capacity were next tested in a more rigorous and physiologic model. Lethally irradiated mice were transplanted with FL cells derived from mice of the various AKT genotypes and then studied for hematopoietic reconstitution. Twelve weeks after the transplantation, AKT1−/−AKT2−/− LSKs were present in equal proportion to control LSKs (Figure 2D). Analysis of the LT-HSC subset showed that AKT1−/−AKT2−/− LT-HSCs comprised a greater fraction of the LSK pool than that found in littermate controls (Figure 2E). The absolute cellularity of the LSK and LT-HSC populations also consistently showed significant differences between the mice reconstituted with AKT1−/−AKT2−/− FL-HSCs compared with mice reconstituted with AKT1+/+AKT2+/− FL-HSCs (data not shown).
Select developmental stages in hematopoiesis may be more dependent on AKT signaling than others for differentiation and proliferation.19,32 Although present in greater numbers, AKT1−/−AKT2−/− LT-HSCs produced fewer mature myeloid and lymphoid cells in the spleen and peripheral blood (Figure 2F; supplemental Figure 3A). In contrast, the erythrocyte lineage was restored similarly to littermate controls (supplemental Figure 3B). The preservation of peripheral blood eosinophils suggests that global myeloid maturation is not dependent on AKT1 and AKT2 (supplemental Figure 3A). These results demonstrate that both AKT isoforms are not required for the reconstitution of LT-HSCs and that the combined absence of these isoforms improves LT-HSC cellularity after a bone marrow transplantation. However, despite the increased presence of LT-HSCs, select hematopoietic lineages fail to develop, such as the lymphoid and myeloid lineages, whereas others, such as the erythroid and eosinophil lineages, are maintained in irradiated mice reconstituted with AKT1/2-deficient cells.
Defective long-term hematopoiesis in the absence of AKT1 and AKT2
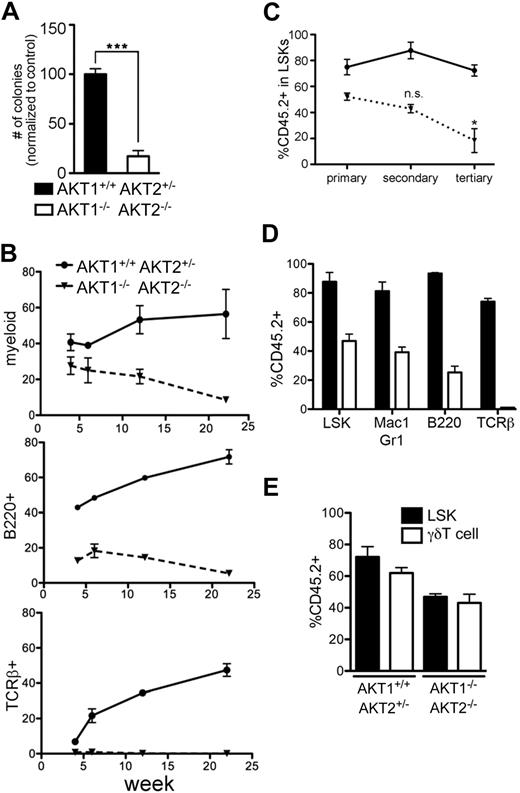
AKT1−/−AKT2−/− FL-HSCs were next tested in long-term functional assays by subjecting them to extended and serial reconstitution studies. The LTC-IC assay is a surrogate in vitro assay for testing LT-HSC function. In the LTC-IC assay, LT-HSCs are maintained in a multipotent state in vitro and can be cultured in differentiation media 6 weeks later to determine whether the LT-HSCs have retained pluripotency. Although LT-HSCs are usually cultured for 6 weeks or longer in LTC-IC assays, we found declined colony-forming capacity in the absence of AKT1 and AKT2 by 2 weeks (Figure 3A). Next, we tested whether AKT1−/−AKT2−/− FL-HSCs would continue to self-renew and differentiate in a competitive environment in vivo. Although injected in a 1:1 ratio with wild-type cells, AKT1−/−AKT2−/− FL-HSCs contributed to a consistently smaller percentage of peripheral blood populations (Figure 3B). Furthermore, AKT1−/−AKT2−/− FL-HSCs were unable to efficiently reconstitute the total LSK compartment after serial secondary and tertiary transplantations in a competitive environment (Figure 3C). Similar results were obtained with the use of adult bone marrow as competitor cells, although adult bone marrow HSCs are less potent stem cells than those derived from FL (supplemental Figure 4).33 Of note, the percentage of AKT1−/−AKT2−/− cells in the splenic myeloid population of each serially transplanted mouse consistently reflected the composition in the LSK compartment, indicating that serial transplantation itself does not adversely affect the differentiation capacity of AKT1−/−AKT2−/− LSKs into mature hematopoietic cells (Figure 3D). The capacity of LSKs to reconstitute the γδT-cell subset was also unaffected by the absence of AKT1 and AKT2 because the percentage chimerism was similar in the LSK and splenic γδT-cell subset (Figure 3E). This result is in contrast to the requirement for AKT1 and AKT2 in αβT-cell lineage development (Figure 3D)19 and further supports the model that AKT1 and AKT2 are required for the development of select lineages.
Trilineage reconstitution by AKT1−/−AKT2−/− HSCs in long-term and serial competitive transplantations. (A) The number of methylcellulose colonies generated from equal numbers of LTC-ICs. LTC-ICs were derived by culturing sorted LSK cells for 2 weeks on OP9 monolayers. Graph represents the mean ± SEM (n = 3) of the number of colonies as a percentage of the control (AKT1+/+AKT2+/−) for each experiment. (B) The percentage of AKT1−/−AKT2−/− (CD45.2+Ly5B6+) cells in the peripheral blood myeloid (Mac-1+Gr-1+), B (B220+), and T (TCRβ+) lineages 4, 6, 12, and 22 weeks after reconstitution. Graphs represent the mean ± SEM (n = 3) for each time point. (C) The percentage of AKT1−/−AKT2−/− (CD45.2+Ly5B6+) cells in the LSK population 16 weeks after the initial transplantation (primary) and after 2 additional 16-week serial transplantations (secondary and tertiary). The graph represents the mean ± SEM (n = 4) of the percentage of CD45.2+Ly5B6+ cells in the LSK population. The AKT1+/+AKT2+/− controls are the same samples represented in Figure 1C. (D) The percentage of AKT1−/−AKT2−/− (CD45.2+Ly5B6+) cells in the bone marrow LSK subset and the splenic myeloid (Mac1+Gr1+), B (B220+), and T (TCRβ+) subsets 16 weeks after the secondary transplantation in the mice from panel C (secondary time point). The graph represents the mean ± SEM (n = 4). The LSK data are the same as in panel C (secondary time point). (E) Splenic γδT-cell competitiveness in the absence of AKT1 and AKT2. γδT cells were identified by gating on the singlet, DAPI−, Thy1+, γδTCR+ population. LSK data are the same as the primary transplantation in panel C. The graph represents the mean ± SEM (n = 4).
Trilineage reconstitution by AKT1−/−AKT2−/− HSCs in long-term and serial competitive transplantations. (A) The number of methylcellulose colonies generated from equal numbers of LTC-ICs. LTC-ICs were derived by culturing sorted LSK cells for 2 weeks on OP9 monolayers. Graph represents the mean ± SEM (n = 3) of the number of colonies as a percentage of the control (AKT1+/+AKT2+/−) for each experiment. (B) The percentage of AKT1−/−AKT2−/− (CD45.2+Ly5B6+) cells in the peripheral blood myeloid (Mac-1+Gr-1+), B (B220+), and T (TCRβ+) lineages 4, 6, 12, and 22 weeks after reconstitution. Graphs represent the mean ± SEM (n = 3) for each time point. (C) The percentage of AKT1−/−AKT2−/− (CD45.2+Ly5B6+) cells in the LSK population 16 weeks after the initial transplantation (primary) and after 2 additional 16-week serial transplantations (secondary and tertiary). The graph represents the mean ± SEM (n = 4) of the percentage of CD45.2+Ly5B6+ cells in the LSK population. The AKT1+/+AKT2+/− controls are the same samples represented in Figure 1C. (D) The percentage of AKT1−/−AKT2−/− (CD45.2+Ly5B6+) cells in the bone marrow LSK subset and the splenic myeloid (Mac1+Gr1+), B (B220+), and T (TCRβ+) subsets 16 weeks after the secondary transplantation in the mice from panel C (secondary time point). The graph represents the mean ± SEM (n = 4). The LSK data are the same as in panel C (secondary time point). (E) Splenic γδT-cell competitiveness in the absence of AKT1 and AKT2. γδT cells were identified by gating on the singlet, DAPI−, Thy1+, γδTCR+ population. LSK data are the same as the primary transplantation in panel C. The graph represents the mean ± SEM (n = 4).
Bone marrow myeloid precursor populations were studied in competitive chimeras to determine whether the loss of AKT1 and AKT2 adversely affected immature myeloid subsets (Figure 4A-B). The surface markers FcγRII/III and CD34 were used to identify the common myeloid progenitor (CMP), the granulocyte-monocyte progenitor (GMP), and the megakaryocyte-erythroid progenitor (MEP) within the lineage-negative, c-Kit–positive subset (Figure 4A). Similar percentages of genetically deficient (CD45.2+) cells were present in the CMP, GMP, and MEP populations compared with the percentage of CD45.2+ cells in the LSK compartment in mice reconstituted with either AKT1/2-deficient or -sufficient cells (Figure 4B). Together, these data indicate that although AKT1 and AKT2 appear dispensable for short-term hematopoiesis and differentiation into select lineages, cells lacking both isoforms compete poorly with their wild-type counterparts in assays requiring LT-HSC self-renewal.
AKT1 and AKT2 are not required for the development of myeloid precursor populations in vivo. (A) Gating scheme used to identify the GMP, CMP, and MEP populations present in the bone marrow of mice that were analyzed 16 weeks after the secondary serial bone marrow transplantation. The populations in panel A are gated on the singlet, DAPI-negative, lineage-negative population (left) and the singlet, DAPI-negative, lineage-negative, c-Kit+ population (right). (B) The percentage of CD45.2+Ly5B6+ cells present in the LSK, CMP, GMP, and MEP populations from the mice described in panel A. LSK data are the same as the secondary transplantation in Figure 3C. The graph represents the mean ± SEM (n = 4).
AKT1 and AKT2 are not required for the development of myeloid precursor populations in vivo. (A) Gating scheme used to identify the GMP, CMP, and MEP populations present in the bone marrow of mice that were analyzed 16 weeks after the secondary serial bone marrow transplantation. The populations in panel A are gated on the singlet, DAPI-negative, lineage-negative population (left) and the singlet, DAPI-negative, lineage-negative, c-Kit+ population (right). (B) The percentage of CD45.2+Ly5B6+ cells present in the LSK, CMP, GMP, and MEP populations from the mice described in panel A. LSK data are the same as the secondary transplantation in Figure 3C. The graph represents the mean ± SEM (n = 4).
Effective differentiation from the LT-HSC to MPP stage requires AKT1 and AKT2
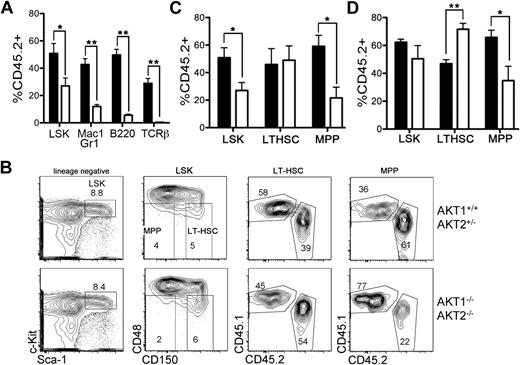
Next, we analyzed the LT-HSC (CD150+CD48−LSK) and MPP (CD150−CD48−LSK) subsets more closely to account for the defective long-term reconstitution with relatively preserved short-term hematopoiesis. Analysis of the percentage chimerism in the CD150+CD48+LSK and CD150−CD48+LSK progenitor subsets generated results that were similar to those from the CD150−CD48−LSK population (data not shown). AKT1−/−AKT2−/− FL-HSCs that were injected into an irradiated host in a 1:1 ratio with congenic FL-HSCs contributed to less than 15% of mature myeloid and lymphoid populations after 6 weeks (Figure 5A). On the basis of the data from 16-week chimeras (Figure 3D), we expected a similar representation of AKT1−/−AKT2−/− LSKs (<15%) as found in the splenic myeloid population of the same animals (Figure 5A). However, analysis of the total LSK population in these mice revealed that AKT1−/−AKT2−/− LSKs comprise approximately 30% of all LSK cells 6 weeks after reconstitution (Figure 5A).
Generation of LT-HSCs and MPPs in a competitive environment. (A) The percentage of AKT1−/−AKT2−/− (CD45.2+Ly5B6+) cells in the BM LSK subset, and the splenic myeloid (Mac-1+Gr-1+), B (B220+), and T (TCRβ+) lineages 6 weeks after reconstitution. Graphs represent the mean ± SEM (n = 3). (B) The gating strategy used to generate the data in panels A, C, and D. Plots are representative of one experiment 6 weeks after reconstitution. (C-D) The percentage of AKT1−/−AKT2−/− (CD45.2+Ly5B6+) cells in the LSK, LT-HSC (CD150+CD48−LSK), or MPP (CD150–CD48−LSK) populations in the bone marrow of mice at either (C) 6 weeks or (D) 12 weeks after reconstitution. Graphs represent the mean ± SEM (n = 3 for panel C and n = 5 for panel D). In panels A through D, FL cells from AKT1−/−AKT2−/− or littermate control animals (CD45.2+Ly5B6+) were mixed with wild-type competitor FL cells (CD45.1+B6.Ly5SJL) in a 1:1 ratio and injected into lethally irradiated CD45.1+B6.Ly5SJL recipients. The LSK data depicted in panels A and C are the same.
Generation of LT-HSCs and MPPs in a competitive environment. (A) The percentage of AKT1−/−AKT2−/− (CD45.2+Ly5B6+) cells in the BM LSK subset, and the splenic myeloid (Mac-1+Gr-1+), B (B220+), and T (TCRβ+) lineages 6 weeks after reconstitution. Graphs represent the mean ± SEM (n = 3). (B) The gating strategy used to generate the data in panels A, C, and D. Plots are representative of one experiment 6 weeks after reconstitution. (C-D) The percentage of AKT1−/−AKT2−/− (CD45.2+Ly5B6+) cells in the LSK, LT-HSC (CD150+CD48−LSK), or MPP (CD150–CD48−LSK) populations in the bone marrow of mice at either (C) 6 weeks or (D) 12 weeks after reconstitution. Graphs represent the mean ± SEM (n = 3 for panel C and n = 5 for panel D). In panels A through D, FL cells from AKT1−/−AKT2−/− or littermate control animals (CD45.2+Ly5B6+) were mixed with wild-type competitor FL cells (CD45.1+B6.Ly5SJL) in a 1:1 ratio and injected into lethally irradiated CD45.1+B6.Ly5SJL recipients. The LSK data depicted in panels A and C are the same.
To more carefully analyze the true contribution of mutant HSCs to the overall HSC pool, the LT-HSC and MPP subsets were analyzed on the basis of their CD150 and CD48 expression (Figure 5B). This more rigorous analysis showed that approximately 50% of the LT-HSC compartment was composed of AKT1−/−AKT2−/− HSCs 6 weeks after injection, but AKT1−/−AKT2−/− HSCs represented no more than 25% to 30% of the MPP population (Figure 5C). A similar analysis of a 12-week chimera showed that the preserved LT-HSC reconstitution and the relative decline in the MPP subset did not normalize over time (Figure 5D). In contrast, AKT1−/−AKT2−/− HSCs became overrepresented (greater than the injected ratio of 1:1) in the LT-HSC compartment (Figure 5D). These data therefore suggest that AKT1 and AKT2 are not required for the initial posttransplantation HSC reconstitution but may be required for efficient transition from the LT-HSC stage to the MPP stage. However, these data do not distinguish between the 2 possibilities that AKT is required for the survival of MPPs or that AKT promotes the differentiation of LT-HSCs into MPPs.
Increased quiescence in AKT1−/−AKT2−/− LT-HSCs
AKT has an impact on major cellular processes, such as survival and proliferation; however, the precise role of these enzymes may depend on cell type and/or stage of differentiation.22,34,35 We speculated that one explanation for the finding of a relatively normal early transplantation response combined with decreased function at later time points could be caused by AKT-dependent proliferation in the LT-HSC compartment. In support of this idea, AKT1−/−AKT2−/− HSCs derived from mice 6 weeks after reconstitution showed cell-cycle distributions similar to controls (supplemental Figure 5A-B). However, after this initial transplantation period, we found a greater proportion of AKT1−/−AKT2−/− LT-HSCs in the G0 stage of the cell cycle when analyzed 12 weeks after transplant (Figure 6A, 6B).36 The proportion of LT-HSCs in the S/G2/M phase of the cell cycle was minimally affected by the combined absence of AKT1 and AKT2 (Figure 6A-B). These data suggest that AKT perturbs the G0 to G1 transition in LT-HSCs after the initial posttransplantation period. This result may also account for the reduced competitiveness in serial transplantation assays found in mice reconstituted with AKT1/2 double-deficient HSCs.
Proliferation and apoptosis in AKT1−/−AKT2−/− LT-HSCs and MPPs. (A-B) The frequency of cells in the G0, G1, and S/G2/M phases of the cell cycle based on RNA (Pyronin Y) and DNA (Hoechst) content. The data in panels A and B are gated on the CD45.2+ LT-HSC (CD150+CD48−LSK) population. The graphs in panel B represent the mean ± SEM (n = 3). (C-D) The frequency of annexin V+ DAPI− cells in the CD45.2+ LT-HSC (CD150+CD48−LSK) or CD45.2+ MPP (CD150−CD48−LSK) compartment. The plots in panel C are gated on the CD45.2+ MPP (CD150−CD48−LSK) population and represent one experiment; the graphs in panel D represent the mean ± SEM (n = 3). Panels A through D are derived from mice reconstituted 12 weeks previously with wild-type (CD45.1+B6.Ly5SJL) and AKT1−/−AKT2−/− or AKT1+/+AKT2+/− FL-HSCs (CD45.2+Ly5B6+).
Proliferation and apoptosis in AKT1−/−AKT2−/− LT-HSCs and MPPs. (A-B) The frequency of cells in the G0, G1, and S/G2/M phases of the cell cycle based on RNA (Pyronin Y) and DNA (Hoechst) content. The data in panels A and B are gated on the CD45.2+ LT-HSC (CD150+CD48−LSK) population. The graphs in panel B represent the mean ± SEM (n = 3). (C-D) The frequency of annexin V+ DAPI− cells in the CD45.2+ LT-HSC (CD150+CD48−LSK) or CD45.2+ MPP (CD150−CD48−LSK) compartment. The plots in panel C are gated on the CD45.2+ MPP (CD150−CD48−LSK) population and represent one experiment; the graphs in panel D represent the mean ± SEM (n = 3). Panels A through D are derived from mice reconstituted 12 weeks previously with wild-type (CD45.1+B6.Ly5SJL) and AKT1−/−AKT2−/− or AKT1+/+AKT2+/− FL-HSCs (CD45.2+Ly5B6+).
Increased apoptosis in AKT1−/−AKT2−/− MPPs
The LT-HSC (CD150+CD48−LSK) to MPP (CD150−CD48−LSK) transition is marked by increased proliferation and a loss of self-renewal capacity.37 We hypothesized that AKT may be required to protect proliferating MPPs from apoptosis.19,34,38 Thus, we compared the frequency of cells undergoing apoptosis (annexin V+ and DAPI−) in the LT-HSC and MPP populations in AKT1−/−AKT2−/− chimeras (Figure 6C-D). The absence of both AKT1 and AKT2 did not affect LT-HSC survival (Figure 6D). In contrast, AKT1−/−AKT2−/− MPPs demonstrated increased apoptosis compared with AKT1+/+AKT2+/− MPPs (Figure 6C-D). Thus, it appears that AKT1 and AKT2 regulate cell survival in the MPP subset but not in the LT-HSC subset.
AKT1 and AKT2 maintain a threshold of intracellular ROS content in LSK cells
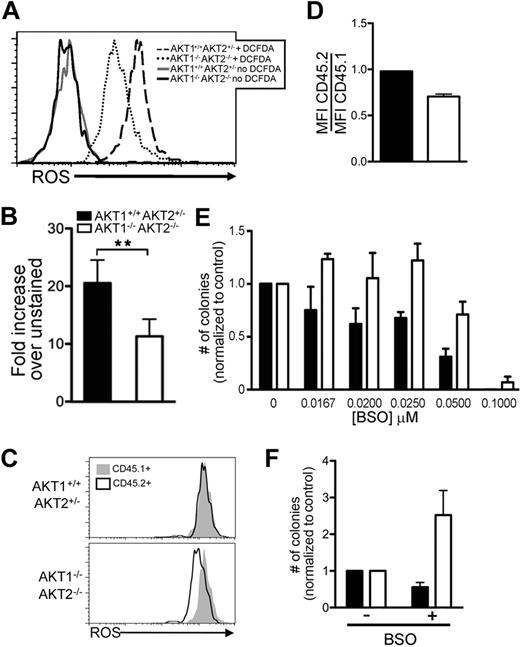
Proliferation in HSCs, like other cell lineages, may be directly linked to the intracellular content of ROS.14,39,–41 We hypothesized that AKT1−/−AKT2−/− LSK cells would have lower ROS content than controls because of the greater population of cells in the G0 phase of the cell cycle. For this assay, we measured ROS levels in freshly isolated LSK cells with 2′-7′-dichlorofluorescene diacetate (DCF-DA; Figure 7A) and found that AKT1−/−AKT2−/− LSK cells had consistently lower ROS content than controls (Figure 7B).
Intracellular ROS in AKT1−/−AKT2−/− LSK cells in vivo and their colony-forming capacity in vitro after pharmacologic increase of ROS. (A-B) ROS content of AKT1−/−AKT2−/− LSK cells. The histogram (A) represents one experiment, and the bar graph (B) represents the mean ± SEM (n = 5) of the MFI of the DCF-DA–treated cells normalized to the HBSS-only–treated cells (no DCF-DA) from each experiment. LSK cells were sorted from bone marrow chimeras, incubated with DCF-DA, and analyzed by flow cytometry. (C-D) ROS content in DN3 thymocytes. Thymocytes from 16-week primary competitive bone marrow chimeras were surface stained with CD4, CD8, CD25, and CD45.2. DN3 staged thymocytes were identified by CD4−CD8−CD25+ phenotype. The histogram (C) represents one experiment, and the bar graph (D) represents the mean ± SEM (n = 3) of the DCF-DA MFI of CD45.2+ cells divided by the DCF-DA MFI of CD45.1+ cells in the same tube. (E) Colony-forming capacity of LSK cells treated with BSO. The graphs represent the total number of colonies found in each culture as a percentage of those generated in the absence of BSO (no BSO = 1) and represent the mean ± SEM (n = 5). Equal numbers of freshly sorted LSK cells were plated in methylcellulose cultures with increasing concentrations of BSO for 10 days before analysis. The number of colonies per plate (mean ± SEM) that used AKT1+/+AKT2+/− cells was 0μM BSO 45 ± 3, 0.0167μM BSO 34 ± 3, 0.020μM BSO 26 ± 2, 0.025μM BSO 29 ± 1, 0.050μM BSO 15 ± 2, and 0.100μM BSO no growth and for AKT1−/−AKT2−/− cells was 0μM BSO 32 ± 2, 0.0167μM BSO 38 ± 1, 0.020μM BSO 32 ± 3, 0.025μM BSO 39 ± 3, 0.050μM BSO 23 ± 2, and 0.100μM BSO 3 ± 1. (F) Effect of BSO treatment on LTC-ICs. LSK cells were grown in LTC-IC cultures for 2 weeks in the presence of 0.025μM BSO. Then, equal numbers of cells were harvested from the LTC-IC cultures and plated in methylcellulose in the absence of BSO for 10 days. The graphs represent the total number of colonies found in each methylcellulose culture as a percentage of those generated in the absence of BSO (no BSO = 1) and represent the mean ± SEM (n = 3).
Intracellular ROS in AKT1−/−AKT2−/− LSK cells in vivo and their colony-forming capacity in vitro after pharmacologic increase of ROS. (A-B) ROS content of AKT1−/−AKT2−/− LSK cells. The histogram (A) represents one experiment, and the bar graph (B) represents the mean ± SEM (n = 5) of the MFI of the DCF-DA–treated cells normalized to the HBSS-only–treated cells (no DCF-DA) from each experiment. LSK cells were sorted from bone marrow chimeras, incubated with DCF-DA, and analyzed by flow cytometry. (C-D) ROS content in DN3 thymocytes. Thymocytes from 16-week primary competitive bone marrow chimeras were surface stained with CD4, CD8, CD25, and CD45.2. DN3 staged thymocytes were identified by CD4−CD8−CD25+ phenotype. The histogram (C) represents one experiment, and the bar graph (D) represents the mean ± SEM (n = 3) of the DCF-DA MFI of CD45.2+ cells divided by the DCF-DA MFI of CD45.1+ cells in the same tube. (E) Colony-forming capacity of LSK cells treated with BSO. The graphs represent the total number of colonies found in each culture as a percentage of those generated in the absence of BSO (no BSO = 1) and represent the mean ± SEM (n = 5). Equal numbers of freshly sorted LSK cells were plated in methylcellulose cultures with increasing concentrations of BSO for 10 days before analysis. The number of colonies per plate (mean ± SEM) that used AKT1+/+AKT2+/− cells was 0μM BSO 45 ± 3, 0.0167μM BSO 34 ± 3, 0.020μM BSO 26 ± 2, 0.025μM BSO 29 ± 1, 0.050μM BSO 15 ± 2, and 0.100μM BSO no growth and for AKT1−/−AKT2−/− cells was 0μM BSO 32 ± 2, 0.0167μM BSO 38 ± 1, 0.020μM BSO 32 ± 3, 0.025μM BSO 39 ± 3, 0.050μM BSO 23 ± 2, and 0.100μM BSO 3 ± 1. (F) Effect of BSO treatment on LTC-ICs. LSK cells were grown in LTC-IC cultures for 2 weeks in the presence of 0.025μM BSO. Then, equal numbers of cells were harvested from the LTC-IC cultures and plated in methylcellulose in the absence of BSO for 10 days. The graphs represent the total number of colonies found in each methylcellulose culture as a percentage of those generated in the absence of BSO (no BSO = 1) and represent the mean ± SEM (n = 3).
The DN3 stage in lymphocyte development may mirror the LT-HSC to MPP transition because both stages require a signal before embarking on the next step in development that is characterized by proliferation with simultaneous differentiation. Thymocyte differentiation past the DN3 stage requires AKT1 and AKT2.19 To determine whether AKT1 and AKT2 are also required to maintain ROS at the DN3 stage, thymocytes from mixed chimeras were surface stained and labeled with DCF-DA (Figure 7C). Similar to LSK cells that lack AKT1 and AKT2, DN3 thymocytes that lack both isoforms (CD45.2+) have decreased ROS compared with same-sample wild-type (CD45.1+) controls (Figure 7C-D). These data support the model that AKT maintains ROS in proliferating cells.42
To determine whether low ROS levels were responsible for functional deficiencies in AKT1−/−AKT2−/− LSK cells, we used BSO to pharmacologically increase ROS in vitro (Figure 7E).43 The ability of AKT1+/+AKT2+/− LSK cells to differentiate in culture was impaired by increasing concentrations of BSO (Figure 7E). In contrast, AKT1−/−AKT2−/− LSK cells demonstrated improved colony-formation capacity when cultured with BSO (Figure 7E). This enhanced colony formation was sustained at the 3 lower dosages of BSO, but greater concentrations of BSO inhibited colony formation even in the absence of AKT1 and AKT2 (Figure 7E).
Because AKT1−/−AKT2−/− FL-HSCs have more pronounced defects in long-term functional assays, we next asked whether pharmacologic modulation of ROS content would affect AKT1−/−AKT2−/− LT-HSC function. In this assay, sorted LSK cells were cultured in the presence of BSO for 2 weeks in LTC-IC cultures. Then, LTC-ICs were harvested and equivalent LTC-ICs were plated in methylcellulose media in the absence of BSO to measure their differentiation capacity (Figure 7F). BSO treatment of AKT1+/+AKT2+/− LSKs had minimal impact on their capacity to form methylcellulose colonies (Figure 7F). In contrast, colony formation by AKT1−/−AKT2−/− LSKs increased with BSO treatment (Figure 7F). Collectively, these data support the model that AKT signaling maintains a threshold of ROS in LSK cells that directly affects overall proliferation and differentiation.
Discussion
By the use of both gain- and loss-of-function approaches, the authors of previous studies9,11,12,19 have documented that signaling via the PI3K pathway is essential for survival, proliferation, and differentiation of HSCs during hematopoietic cell development. However, the role of AKT, a key effector of the PI3K signaling cascade, in HSC function had not been thoroughly explored. In this report, we examined the importance of AKT1 and AKT2, the 2 AKT isoforms expressed at highest levels in hematopoietic cells, for LT-HSC and MPP activity. Our initial approach was to evaluate the role of these 2 enzymes individually by analyzing mice deficient in one or the other isoform for HSC functionality and found little to no impact of loss of either single isoform. This observation is reminiscent of other experimental systems in which the actions of AKT isoforms appear to overlap, requiring study of animals with compound deficiencies to fully analyze the contribution of AKT for critical cellular functions.19,22,44
We therefore examined the impact of loss of both AKT1 and AKT2 in hematopoietic reconstitution experiments. Unexpectedly, AKT1−/−AKT2−/− LT-HSCs (CD150+CD48−LSK) accumulated in greater numbers and appeared to outcompete wild-type cells during the reconstitution phase of an irradiated host. However, upon analysis of the MPP (CD150−CD48−LSK) compartment, we found a significant underrepresentation of cells lacking the 2 AKT isoforms. These data suggest that double-deficient cells are competent during the initial proliferation stage to fill the HSC niches but reveal defects after differentiation into the MPP population. Following the model that LT-HSC space is limited, our data suggest that AKT1−/−AKT2−/− LT-HSCs are initially superior to wild-type cells in competition for this space. However, although the more quiescent AKT1/2-deficient cells may have a greater niche occupancy rate initially, during more extended time periods, this quiescence is detrimental to AKT1−/−AKT2−/− LT-HSCs because they are less efficient at self-renewal and are eventually outcompeted by wild-type LT-HSCs.
We considered 2 nonmutually exclusive possibilities to explain selective loss of MPPs in the setting of AKT deficiency: failure of the LT-HSCs to differentiate into MPPs and relative susceptibility of AKT-deficient MPPs to undergo spontaneous apoptosis compared with their wild-type counterparts. Our data suggest that the paucity of AKT1−/−AKT2−/− MPPs can be explained, at least in part, by both mechanisms. First, we found that the doubly deficient LT-HSCs were more quiescent than wild-type cells, with many fewer entering cell cycle as measured by DNA and RNA content. Second, we found that those MPPs that did develop in the absence of the 2 AKT isoforms had a greater rate of spontaneous apoptosis than wild-type MPPs, as measured by annexin V staining immediately after recovery from their hosts.
The metabolic demands on the relatively proliferative MPP subset makes cells at this stage of development more susceptible to cell death. Thus, the finding that AKT-deficient MPPs demonstrate increased cell death is consistent with the established role of AKT to protect cells under stress from apoptosis.38 This observation is also consistent with similar data from T-cell development studies showing decreased competitiveness in proliferative populations that lack both AKT1 and AKT2 but not their more quiescent counterparts.19
Although we are not certain about how AKT exerts its effects on LT-HSC proliferation, similar to findings in other lineages, a link between intracellular oxidation status and cellular proliferation has been established in HSCs.45,46 Juntilla et al19 showed that AKT1−/−AKT2−/− DN3 thymocytes were less metabolically active than littermate controls. We therefore examined the impact of AKT loss on ROS content in HSC subsets and at the DN3 thymocyte stage. This investigation was of particular interest, given previous work that established diminished HSC serial transplant repopulation capacity with aging, a feature that has been causally associated with an increased intracellular oxidation state.45,47 We therefore speculated that AKT loss might result in greater than normal levels of ROS, thus interfering with hematopoietic lineage development. In contrast, we found that ROS levels were lower in the mutant LSK and DN3-staged T cells. Pharmacologic increases of ROS levels, although (as expected) toxic to wild-type LSK cells, restored the proliferative and differentiation capacity of the AKT1/2 double-deficient LSK cells.
These data indicate that LSK function requires sufficient ROS levels, presumably to drive cells into cycle, but that excessive ROS diminishes LSK cell activity.45 AKT appears critical to maintain this fine balance of ROS levels necessary for optimal HSC proliferation and hematopoietic cell differentiation and extends the finding that not only are too high levels of ROS toxic to HSCs but sufficient ROS are also required for normal function. These data are concordant with a recent study42 in which the authors demonstrated that AKT1−/−AKT2−/− mouse embryonic fibroblasts also have decreased ROS and reduced proliferation. Regulation of ROS by AKT was shown further to control fibroblast cell senescence. It will be an important area of investigation to determine whether AKT also regulates HSC senescence through control of ROS levels.
It is intriguing that AKT deficiency lowers basal ROS levels in HSCs. Endogenous sources of ROS include the nicotinamide adenine dinucleotide phosphate-oxidase complex, peroxisomes, and mitochondrial respiration.40 Brunet et al13 showed that AKT inhibits the activity of members of the FOXO family of transcription factors. Because FOXO is known to regulate genes whose products down-regulate ROS (for example, catalase and superoxide dismutase, members of the antioxidant system),48 we speculated that AKT loss would lead to increased FOXO function and up-regulation of transcripts for these ROS regulators. In multiple experiments, however, we were unable to find any difference in Catalase or Sod2 mRNA in AKT1−/−AKT2−/− HSCs (data not shown). Alternatively, HSCs may regulate ROS through different mechanisms than those found in other cell lineages, such as through an AKT-dependent but FOXO-independent activation of the mitochondrial electron transport chain.49,50
ROS have been implicated as a second messenger system that directly regulates proliferation.40,45,51 Our data support the model that the PI3K/AKT/FOXO pathway is one of the major regulators of ROS-induced cell proliferation and hematopoietic differentiation. In the hypoxic HSC niche model,52 physiologic exposure to increasing amounts of ROS may induce HSCs to proliferate in the niche. Upon activation, HSCs may leave the hypoxic niche and relocalize to a region with greater exposure to exogenous oxygen and elevated ROS. It would be important to determine whether AKT1−/−AKT2−/− LSKs localize to bone marrow niches that are exposed to more environmental ROS than wild-type LSKs, thus compensating for their basally decreased intracellular ROS content.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Drs Mitch Weiss (Children's Hospital of Philadelphia) and Ivan Maillard (University of Michigan) for critically reviewing the manuscript. We also gratefully acknowledge the expert technical support in flow cytometry provided by Ryan Wychowanec and all other staff members of the Abramson Cancer Center Flow Cytometry and Cell Sorting Shared Resource. We thank Dr Suresh Shelat of the Children's Hospital of Philadelphia Hematology Laboratory for assistance with peripheral blood counts.
This work was supported by National Institutes of Health grants R37-GM053526 and P01-CA093615 (G.A.K.), R01-DK56886 (M.J.B.), and F31-AI056671 (M.M.J.).
National Institutes of Health
Authorship
Contribution: M.M.J. designed experiments, performed research, analyzed data, and wrote the manuscript; V.D.P., M.C., and R.P.J. performed research; M.J.B. provided key reagents and analyzed data; and G.A.K. designed experiments, analyzed data, and supervised the project.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Gary A. Koretzky, University of Pennsylvania, 415 BRBII/III, 421 Curie Blvd, Philadelphia, PA 19104; e-mail: Koretzky@mail.med.upenn.edu.