Abstract
Establishment of a system with efficient generation of natural killer T (NKT) cells from embryonic stem (ES) cells would enable us to identify the cells with NKT-cell potential and obtain NKT cells with desired function. Here, using cloned ES (NKT-ES) cells generated by the transfer of nuclei from mature NKT cells, we have established a culture system that preferentially developed functional NKT cells and also identified early NKT progenitors, which first appeared on day 11 as a c-kit+ population in the cocultures on OP9 cells with expression of Notch ligand, delta-like1 (OP9/Dll-1) and became c-kitlo/− on day 14. Interestingly, in the presence of Notch signals, NKT-ES cells differentiated only to thymic CD44lo CD24hi NKT cells producing mainly interleukin-4 (IL-4), whereas NKT cells resembling CD44hi CD24lo liver NKT cells producing mainly interferon γ (IFN-γ) and exhibiting strong adjuvant activity in vivo were developed in the switch culture starting at day 14 in the absence of Notch. The cloned ES culture system offers a new opportunity for the elucidation of the molecular events on NKT-cell development and for the establishment of NKT-cell therapy.
Introduction
Natural killer T (NKT) cells are characterized by their expression of an invariant receptor encoded by Vα14-Jα18 in mice1 and by Vα24-Jα18 in humans.2,3 Because the invariant receptor is used only by NKT cells and not by conventional T cells, NKT cells are a distinct lineage from conventional T cells. NKT cells produce both T helper 1 (Th1) and Th2 cytokines, mediating strong adjuvant activity through their Th1 cytokine production essential for protective responses against tumors4-6 and pathogens7-9 and also protecting autoimmune disease development through their Th2 cytokine production.10,11 Despite the importance of this cell type in the immune system, the identity of NKT progenitor cells and their subsequent development remain poorly understood. In previous reports, cells with NKT-cell potential were detected in the CD4CD8 double-positive (DP) thymocyte population,12,13 indicating that NKT cells are branched off from conventional αβT-cell precursors at the DP stage in the thymus.12-14 It has also been shown that the early immature CD4 NKT cells produce only interleukin-4 (IL-4), but their potential to produce interferon γ (IFN-γ) is acquired at a later developmental stage.15,16 However, it is still possible that NKT cells might be derived from a precursor population distinct from that of conventional αβT cells, because it has previously been shown that NKT precursor cells express on their cell surface granulocyte-macrophage colony-stimulating factor receptor (GM-CSFR),17 which is known to be a unique marker for myeloid cell lineages but not for lymphoid cells. In addition, because the invariant Vα14 receptor is used only by NKT cells and not by conventional T cells, NKT cells may be a distinct lineage from conventional αβT cells.18
Embryonic stem (ES) cells are a powerful model system in which to study in vitro lymphocyte differentiation, addressing the questions on the cells with NKT precursor potential and the ability of NKT cells to produce both Th1 and Th2 cytokines during their development. For example, embryoid bodies generated from ES cells contain CD34+ cells that develop into lymphocytes when cocultured with OP9 stromal cells plus appropriate cytokines.19 However, in most cases, ES cells generate B and NK cells, but not T or NKT cells on OP9 coculture.20,21
To overcome these problems, OP9 stromal cells transduced with Notch ligand delta-like1 (OP9/Dll-1) are used for the directed differentiation of ES cells to T-cell lineages. Induction of Notch signals directs stem cells to differentiate into immature DP T cells and inhibits B-cell development, indicating that Notch signaling is required as a proximal event in T-cell commitment from progenitors.22,23
Another approach is to use cloned ES (NKT-ES) cells established by nuclear transfer of a cell with a rearranged T-cell receptor (TCR) gene.24,25 Interestingly, the cells that retain high genome reprogrammability are NKT cells (71% for NKT vs 12% for T cells).24 Therefore, the NKT-cell nucleus should provide important clues for analysis of differentiation pathways from ES cells to mature NKT cells.
In the present study, we have established NKT-ES cells generated by nuclear transfer of C57BL/6 (B6) NKT cells, and also an in vitro culture system, in which we identified the cells with NKT-cell potential as a homogenous population and generated Th2-like NKT cells on OP9/Dll-1 and also Th1-like NKT cells in the switch culture starting at day 14 of culture on OP9 cells without Notch signaling (OP9/control).
Methods
Mice
B6, B6D2F1, and ICR mice (6-8 weeks old) were purchased from Charles River Japan Inc or CLEA Japan Inc. TAP−/− female mice (6-8 weeks old) were purchased from The Jackson Laboratory. Jα18−/− mice were generated previously7 and backcrossed for more than 10 generations to B6 mice. Mice were kept under specific pathogen-free conditions and used at 8 to 16 weeks of age. All experiments were done in accordance with protocols approved by RIKEN Animal Care and Use Committee.
NKT-ES cell lines
B6 male mice, 8 to 16 weeks old, were used as nuclear donors for direct nuclear transfer experiments. NKT cells were prepared from liver mononuclear cells as previously described.26 The purity of NKT cells sorted twice by fluorescence-activated cell sorting (FACS) was more than 99%, with 97% to 99% viability. Nuclear transfer was performed as described previously27 using 8-week-old B6D2F1 female mice as oocyte donors. Reconstructed embryos were cultured in potassium-enriched simplex optimized medium28 supplemented with 100mM ethylenediaminetetraacetic acid to develop the blastocyst stage. For the efficient generation of NKT-ES cell lines from the B6 strain, a serial nuclear transfer approach was applied.29 In brief, in vivo–fertilized 2-cell–stage (B6D2F1 × ICR)F1 embryos served as secondary cytoplasm donors. Blastocysts obtained by serial cloning were transferred to 4-well plates on mouse embryonic fibroblast feeder cells in ES derivation medium (CultiCell) supplemented with 1000 U of leukemia inhibitory factor (R&D Systems) and 50μM 2-mercaptoethanol. After 7 to 10 days of initial growth, expanded inner cell masses were mechanically split into several groups of cells, cultured for an additional 3 to 4 days, and then passaged after treatment with a trypsin–ethylenediaminetetraacetic acid solution (Invitrogen). NKT-ES cells generated were maintained on irradiated mouse embryonic fibroblasts prepared from day-15 to -18 embryos as described27 in ES cell medium (Dulbecco modified Eagle medium supplemented with 15% fetal calf serum, 10 U/mL penicillin, 100 μg/mL streptomycin, 100 μg/mL gentamicin, 2mM glutamine, 110 μg/mL sodium pyruvate, 50μM 2-mercaptoethanol, and 10mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) containing 1 ng/mL leukemia inhibitory factor. NKT-ES-V cells were generated from NKT-ES clone 3 by introducing the internal ribosome entry site (IRES)–Venus in-frame targeting vector downstream of the TCR Cα stop codon as previously described.7 ES cells were generated from B6 mice. Primers used are shown in supplemental Table 1 (available on the Blood website; see the Supplemental Materials link at the top of the online article).
NKT-ES culture
OP9/control and OP9/Dll-1 (generated by transfection of a retroviral vector containing a Dll-1 cDNA) were used as a monolayer with OP9 medium (α-minimum essential medium supplemented with 20% fetal calf serum [HyClone], 10 U/mL penicillin, 100 μg/mL streptomycin, and 2.2 g/L sodium bicarbonate). The method to generate NKT cells is the same as a published protocol.30 In brief, NKT-ES cells (5 × 104) were cultured with OP9/Dll-1 as an adherent cell layer in OP9 medium. On day 6 of culture, most NKT-ES colonies were disrupted by treatment with 0.25% trypsin (Invitrogen). The nonadherent cells were then cultured for another 4 days on a fresh OP9/Dll-1 in OP9 medium with the addition of Flt-3 ligand (Flt3L; 5 ng/mL; Peprotech). On day 10 of culture and every 4 days thereafter, nonadherent NKT-ES–derived hematopoietic cells were cultured on a fresh OP9/Dll-1 in OP9 medium with Flt3L (5 ng/mL; Peprotech) and IL-7 (5 ng/mL; Peprotech). In some experiments, cells obtained at the indicated days of the NKT-ES/OP9/Dll-1 cultures were further cocultured on OP9/control. The NKT-ES culture system described is similar to a published protocol.31
Generation of anti–GM-CSFRα mAb
A GM-CSFRα–immunoglobulin (Ig) fusion gene was constructed by fusing the cDNA of the extracellular domain of GM-CSFRα in frame to the CH2-CH3 domains of human IgG1 in the pIRES2–enhanced green fluorescent protein expression vector (Clontech). GM-CSFRα–Ig was purified from the culture supernatants of transfected HEK293 cells using a protein A–Sepharose column as previously described32 (GE Healthcare; supplemental Figure 1A). Anti–GM-CSFRα monoclonal antibody (mAb; 3A6D1) was developed by immunization of Wister rats with GM-CSFRα–Ig. After initial screening by enzyme-linked immunosorbent assay on the GM-CSFRα–Ig fusion protein, 100 hybridoma clones were further characterized by flow cytometry on GM-CSFRα and GM-CSFRα/commonβ transfectants (supplemental Figure 1B). The specificity of 3A6D1 was shown in supplemental Figure 1C and D, demonstrating that the antibody reacted with GM-CSFRα+ conventional dendritic cells, but not other cell types, including CD4+ T cells, CD8+ T cells, or plasmacytoid dendritic cells.
Flow cytometry
Biotin-, fluorescein isothiocyanate–, phycoerythrin-, phycoerythrin–cyanin 7–, allophycocyanin-, allophycocyanin—cyanin 7–, or Pacific Blue–conjugated mAbs were purchased from BD Biosciences or eBioscience and are listed in supplemental Table 1. Flow cytometry was performed using a FACSCalibur and FACSAria (BD) with FlowJo software (TreeStar). The purity of sorted cells was usually more than 99%.
PCR analysis
Genome was isolated from cells using Gentra Puregene kit (QIAGEN) for genotyping. Genomic polymerase chain reaction (PCR) was performed with LA Taq (Takara). Primers used are listed in supplemental Table 2. Total RNA was isolated from FACS-purified cell populations using the TRIzol reagent (Invitrogen) for reverse-transcription (RT)–PCR analysis. RT-PCR was performed with the Superscript III 1-step RT-PCR with PlatinumTaq kit (Invitrogen). Gene-specific primer sequences are provided in supplemental Table 3.
Cytokine and proliferation assays
NKT cells generated on OP9/Dll-1 or OP9/control were cocultured with GM-CSF–induced bone marrow–derived dendritic cells33 for 3 days in 96-well U-bottom plates. For cytokine assays, the culture supernatants were analyzed by a cytometric bead array (BD Biosciences) according to the manufacturer's protocol. For proliferation assays, cells were pulsed with 0.037 MBq/well of [3H]thymidine (GE Healthcare) for the last 16 hours. Radioactivity was measured using the MicroBeta instrument (PerkinElmer). Average of triplicate and standard deviation is shown.
Adjuvant activity of NKT cells derived from NKT-ES cells
The adjuvant activities of NKT cells generated in vitro were assayed as described previously.34 Briefly, spleen cells from TAP−/− mice were incubated with hypertonic medium in the presence of 10 mg/mL ovalbumin (OVA) and then incubated with hypotonic medium to induce apoptosis. This cell-associated form of OVA was injected intravenously (2 × 107 cells/mouse together with α-galactosylceramide [α-GalCer; 2 μg/mouse], which is termed TOG [TAP−/−/OVA/α-GalCer] immunization) into B6 wild-type mice or Jα18−/− mice on a B6 background that had received in vitro–generated NKT cells (106 cells) 1 hour before TOG immunization. After 7 days, spleen cells were stimulated in vitro with 1μM OVA257-264 peptide for 6 hours, and then IFN-γ production was monitored by intracellular staining. For intracellular cytokine staining, Brefeldin A was added for the last 4 hours of culture to accumulate intracellular cytokines. Cells were then washed and incubated with anti–mouse CD8 mAb for 20 minutes at 4°C after first blocking Fc receptors with an anti-CD16/CD32 antibody. After fixation with Cytofix/Cytoperm plus (BD Biosciences), cells were stained for intracellular IFN-γ for 15 minutes at room temperature.
Results
Because rearranged transgenic TCRs derived from NKT cells predispose to the NKT fate in vivo,35,36 we attempted to generate NKT-ES cells by nuclear transfer of mature NKT cells with the idea that the prerearranged invariant Vα14-Jα18 gene, the defining marker of NKT cells, in its natural chromosomal context would provide advantages to investigate NKT-cell development in the early stages and also to generate functional NKT cells in vitro. Although NKT-cell cloned mice and derivative ES cell lines have been established using (B6 × 129)F1 NKT-cell nuclei, their mixed genetic background causes multiple problems in phenotypic analyses and cell transfer experiments.25 Therefore, we decided to generate NKT-ES cells on a genetically homogeneous B6 background.
Establishment of NKT-ES cell lines by transfer of NKT-cell nucleus
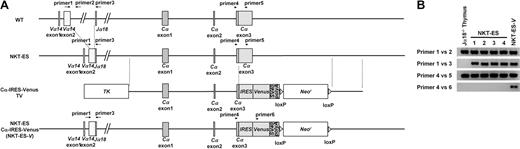
B6 ES clones had a very low success rate because of poor developmental capacity to the blastocyst, which is a pre–ES-cell stage. However, we finally established NKT-ES cell lines by nuclear transfer of B6 liver NKT cells into B6D2F1 unfertilized eggs followed by retransfer of 2-cell–stage nuclei into the 2-cell–stage cytoplasm of (B6D2F1 × ICR)F1 in vivo–fertilized embryos. In total, 6 blastocysts were obtained from the 147 initial 2-cell–stage embryos. This efficiency (4%) is similar to that reported for clones from cumulus cells (2.3%-6.9%) or adult fibroblasts (1.1%-3.8%).25 Four of 6 cell lines established were analyzed by genomic PCR using primers that detect the non-rearranged Vα14 gene and primers that specifically detect the Vα14-Jα18 gene rearrangement (Figure 1A). This analysis demonstrated that all established NKT-ES clones contained the rearranged genomic Vα14-Jα18 on one chromosome and the germline configuration on the other (Figure 1B). We used clone 3 throughout these experiments (Figure 1B lane 4).
Generation of NKT-ES and NKT-ES-V cells by direct nuclear transfer of mature NKT cells. (A) Schematic representation of the TCRα locus in natural killer T embryonic stem (NKT-ES) and NKT-ES-V cells. The NKT-ES cells generated by nuclear transfer of B6 liver NKT cells were prepared as described elsewhere,27,28 with a slight modification. NKT-ES-V cells were generated by transfection of the Cα-IRES-Venus targeting vector, which placed the Venus cDNA, along with its upstream IRES, downstream of TCRα locus adjacent to the Cα stop codon. (B) Genomic polymerase chain reaction (PCR) of NKT-ES and NKT-ES-V cells. Primer pairs shown in panel A were used for genomic PCR. Primer sequences are listed in supplemental Table 2. Lane 4 is clone 3, which was used in the present study.
Generation of NKT-ES and NKT-ES-V cells by direct nuclear transfer of mature NKT cells. (A) Schematic representation of the TCRα locus in natural killer T embryonic stem (NKT-ES) and NKT-ES-V cells. The NKT-ES cells generated by nuclear transfer of B6 liver NKT cells were prepared as described elsewhere,27,28 with a slight modification. NKT-ES-V cells were generated by transfection of the Cα-IRES-Venus targeting vector, which placed the Venus cDNA, along with its upstream IRES, downstream of TCRα locus adjacent to the Cα stop codon. (B) Genomic polymerase chain reaction (PCR) of NKT-ES and NKT-ES-V cells. Primer pairs shown in panel A were used for genomic PCR. Primer sequences are listed in supplemental Table 2. Lane 4 is clone 3, which was used in the present study.
Establishment of an in vitro NKT-ES culture system
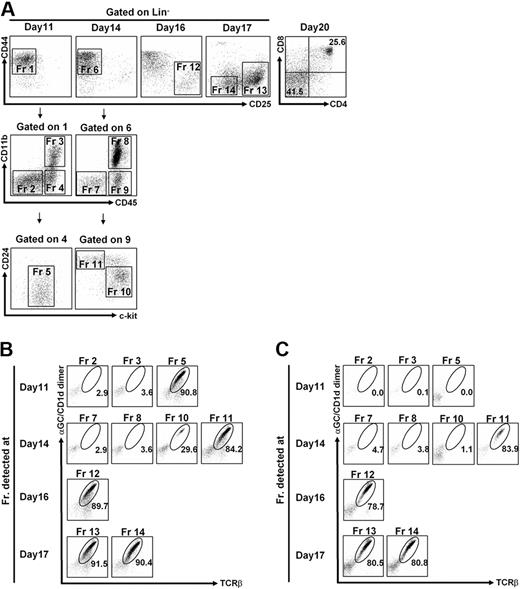
Using NKT-ES cells, we established an NKT-ES culture system using OP9/Dll-1 as an adherent cell layer in the presence of IL-7 and Flt3L. Cells generated in vitro were analyzed for surface phenotypes at indicated time points after cultivation (Figure 2A). In the T-cell development, pre-TCR signaling is essential for differentiation of CD4−CD8− double-negative (DN) cells to the DP stage. The DN cells are subdivided into DN1-DN4 subsets, according to the expression of CD44 and CD25. The earliest CD44+CD25− (DN1) cells provide the CD44+CD25+ (DN2) population that progresses to the CD44−CD25+ (DN3) stage, followed by the CD44−CD25− (DN4) stage. NKT-ES cells were sustained in culture as DN1 population from day 11 to day 14, which was separated by 3 fractions (Fr) according to the expression of CD45 and CD11b. As shown in Figure 2A, the CD45+/CD11b− subpopulation expressed an intermediate level of c-kit at day 11 (Fr 5), whereas the CD45+/CD11b− population consisted of 2 fractions on day 14 with phenotype of CD24+/c-kitlo/− (Fr 11) and CD24dull/c-kithi (Fr 10). On the following days of culture, cells displayed phenotypes similar to the thymic DN2/3 population (CD44lo/−CD25hi; Fr 12) by day 16, subsequently into DN3/4 cells (CD44− CD25−/int; Fr 14) on day 17. Finally, DP cells appeared on day 20 of culture, most of which were α-GalCer/CD1d dimer+/TCRβ+ NKT cells (Figure 2A). Therefore, it seems that the NKT-ES culture system populated NKT cells in vitro, tracing similar developmental progression as detected in the unmanipulated ES culture system as well as in thymocytes in vivo.39
In vitro NKT-ES cell culture. (A) Fluorescence-activated cell sorting (FACS) profiles of cells generated from NKT-ES cells. Cells generated from NKT-ES cells on OP9/Dll-1 in the presence of IL-7 and Flt3L were analyzed by FACSAria at the indicated time points. On days 11 and 14 of culture, Lin (CD3ϵ, CD4, CD8α, TCRβ, TCRγδ, α-GalCer/CD1d dimer, CD11c, CD19, B220, Gr-1)− CD44+ CD25− cells, equivalent to the thymic DN1 population, were further separated based on CD45 and CD11b expression. The CD45+ CD11b− cells were then analyzed for expression of c-kit and CD24. (B-C) Generation of NKT cells from NKT-ES cells. Indicated fractions in panel A were sorted and further cultured on OP9/Dll-1 with IL-7 and Flt3L for a total of 20 days (B), or in the switch culture on OP9/control with IL-7 and Flt3L starting from day 14 of the original culture for the following 6 days (C). The α-GalCer/CD1d dimer+ TCRβ+ fraction generated from OP9/Dll-1 (B) or from switch culture on OP9/control (C) was analyzed by FACS.
In vitro NKT-ES cell culture. (A) Fluorescence-activated cell sorting (FACS) profiles of cells generated from NKT-ES cells. Cells generated from NKT-ES cells on OP9/Dll-1 in the presence of IL-7 and Flt3L were analyzed by FACSAria at the indicated time points. On days 11 and 14 of culture, Lin (CD3ϵ, CD4, CD8α, TCRβ, TCRγδ, α-GalCer/CD1d dimer, CD11c, CD19, B220, Gr-1)− CD44+ CD25− cells, equivalent to the thymic DN1 population, were further separated based on CD45 and CD11b expression. The CD45+ CD11b− cells were then analyzed for expression of c-kit and CD24. (B-C) Generation of NKT cells from NKT-ES cells. Indicated fractions in panel A were sorted and further cultured on OP9/Dll-1 with IL-7 and Flt3L for a total of 20 days (B), or in the switch culture on OP9/control with IL-7 and Flt3L starting from day 14 of the original culture for the following 6 days (C). The α-GalCer/CD1d dimer+ TCRβ+ fraction generated from OP9/Dll-1 (B) or from switch culture on OP9/control (C) was analyzed by FACS.
Cells with NKT-cell potential appear at an early stage in the NKT-ES cultures
To investigate which fractions developed in the OP9/Dll-1 coculture system (Fr 2 to Fr 14, Figure 2A) have NKT-cell potential, each faction obtained at the indicated time point was purified by FACS and recultured in the OP9/Dll-1 coculture system for 20 days for the first culture. We found that cells in Fr 5 detected at day 11, and in Fr 10 to Fr 14 at day 14 to day 17 of culture, gave rise α-GalCer/CD1d dimer+, TCRβ+ NKT cells at day 20 of culture, suggesting that these fractions contained cells with NKT-cell potential. However, other fractions, such as Fr 2, Fr 3, Fr 7, and Fr 8, failed to develop into NKT cells (Figure 2B). Thus, the earliest cells with NKT-cell potential are enriched in CD44hi/CD25−/CD24dull/c-kit+/dull population from days 11 to 14 of cultivation (Figure 2A).
To investigate the role of Notch signaling in NKT-cell development, cells obtained at indicated points from the OP9/Dll-1 (Notch-signaling dependent) culture were subsequently cocultured on OP9/control (switch culture). As shown in Figure 2C, α-GalCer/CD1d dimer+ NKT cells were successfully generated from Fr 11 to Fr 14, but not Fr 5, with NKT-cell potential on OP9/Dll-1 (Figure 2B). The result indicates that NKT cells do develop in a Notch-independent fashion from Fr 11 (at day 14) but not Fr 5 (at day 11), indicating that Notch signaling is required for the development of NKT cells in the early stage, but is not essential in the late stage after day 14 of culture. It is important to mention that Fr 10 also contains the cells with NKT-cell potential, but their potential is limited and distinct from Fr 11, because the cells in Fr 10 require Notch signaling for their development (Figure 2B-C). Of importance, the NKT-ES culture system developed mainly NKT cells but not other lymphoid cell lineages, such as αβT, γδT, B, and NK cells, except for CD11b+ cells, on either OP9/control or OP9/Dll-1 (supplemental Figure 2). Interestingly, B cells were not developed by OP9/control cultures of NKT-ES cells, in contrast to wild-type (WT) ES cells with OP9/control cocultures, indicating the possibility that prearrangement of TCR locus in NKT-ES cells may affect B-cell development (supplemental Figure 2). Moreover, NKT-cell development was not detected in the culture of unmanipulated ES (supplemental Figure 3) or cloned ES cells generated by nuclear transfer of conventional T cells (Y.N. and M.T., unpublished observations, February 2009), indicating that the rearranged invariant Vα14 TCR expression in the early developmental stage predisposes the fate of NKT cells.
Early NKT-cell progenitors identified in the NKT-ES culture
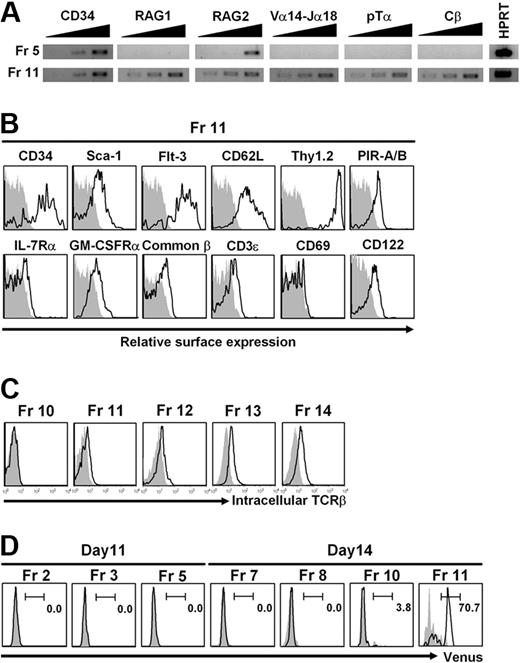
The cells in Fr 5 and Fr 11 with NKT-cell potential are likely to represent 2 waves of NKT progenitors. The cells in Fr 5 expressed RAG-2 transcripts, but were negative for Vα14-Jα18, Cβ, and RAG-1 transcripts. On the other hand, the cells in Fr 11 expressed RAG-1/RAG-2, pre–TCR alpha (pTα), Vα14-Jα18, and Cβ transcripts (Figure 3A), but did not express an invariant Vα14 receptor on the surface (supplemental Figure 4). The surface phenotypes of the cells in Fr 11 were CD34+, c-kitlo/−, Thy1+, Flt-3+, GM-CSFRα+, PIR-A/B+, and CD69− (Figure 3B), distinct from those of DP thymocytes or NKT cells in the liver (supplemental Figure 5) and also different from those of NKT cells generated in vitro from NKT-ES (Figure 4). We were unable to determine the phenotype of the cells with NKT-cell potential in Fr 5 because of limited cell numbers. To further characterize the cells in Fr 11, we performed intracellular TCRβ staining of Fr 10 to Fr 14 (Figure 3C). The cells in Fr 11 to Fr 14, but not in Fr 10, already possess TCRβ expression, and thus have NKT-cell developmental potential.
Analysis of the cell fraction with NKT-cell potential. (A) Reverse-transcription (RT)–PCR analysis. The cells in Fr 5 and Fr 11 shown in Figure 2A were sorted, and total RNA was isolated. The indicated genes were analyzed by RT-PCR using primer pairs listed in supplemental Table 3. Total RNA from 5 × 102, 1 × 103, and 2 × 103 cell equivalents was used as template in each lane. Hypoxanthine guanine phosphoribosyl transferase (HPRT) was used as a loading control and the RNA was from 5 × 102 cells. (B) Surface phenotypes. The cells in Fr 11 shown in Figure 2A were analyzed for surface markers by flow cytometry. Shadowed profiles indicated isotype-matched control antibody staining. (C) Intracellular TCRβ staining. The cells in Fr 10 to Fr 14 shown in Figure 2A were analyzed for intracellular TCRβ expression by flow cytometry. Shadowed profiles indicated isotype-matched control antibody staining. (D) FACS analysis of cells derived from NKT-ES-V cells. The developmental progression of NKT-ES-V cells cultured on OP9/Dll-1 under the same conditions as shown in Figure 2A was the same as that of NKT-ES. Thus, cell fractions equivalent to Fr 2, Fr 3, and Fr 5 on day 11 and Fr 7, Fr 8, Fr 10, and Fr 11 on day 14 of culture shown in Figure 2A were gated and analyzed for Venus expression by FACSAria.
Analysis of the cell fraction with NKT-cell potential. (A) Reverse-transcription (RT)–PCR analysis. The cells in Fr 5 and Fr 11 shown in Figure 2A were sorted, and total RNA was isolated. The indicated genes were analyzed by RT-PCR using primer pairs listed in supplemental Table 3. Total RNA from 5 × 102, 1 × 103, and 2 × 103 cell equivalents was used as template in each lane. Hypoxanthine guanine phosphoribosyl transferase (HPRT) was used as a loading control and the RNA was from 5 × 102 cells. (B) Surface phenotypes. The cells in Fr 11 shown in Figure 2A were analyzed for surface markers by flow cytometry. Shadowed profiles indicated isotype-matched control antibody staining. (C) Intracellular TCRβ staining. The cells in Fr 10 to Fr 14 shown in Figure 2A were analyzed for intracellular TCRβ expression by flow cytometry. Shadowed profiles indicated isotype-matched control antibody staining. (D) FACS analysis of cells derived from NKT-ES-V cells. The developmental progression of NKT-ES-V cells cultured on OP9/Dll-1 under the same conditions as shown in Figure 2A was the same as that of NKT-ES. Thus, cell fractions equivalent to Fr 2, Fr 3, and Fr 5 on day 11 and Fr 7, Fr 8, Fr 10, and Fr 11 on day 14 of culture shown in Figure 2A were gated and analyzed for Venus expression by FACSAria.
Analysis of NKT cells derived from NKT-ES cells. (A) FACS profiles of in vitro–generated NKT cells. Cells developed from NKT-ES cells on day 20 of culture with OP9/Dll-1 (Th2-like NKT) or by the switch culture using OP9/control (Th1-like NKT) were gated on the α-GalCer/CD1d dimer+ TCRβ+ fraction and further analyzed for the expression of the indicated markers, NK1.1 versus CD3ϵ and CD4 versus CD8. (B) Surface phenotypes of in vitro–generated NKT cells. Gated fractions shown in panel A were further analyzed for the expression of the indicated markers. Shadowed profiles indicate isotype-matched control staining. (C) Proliferation and cytokine production of in vitro–generated NKT cells upon stimulation with α-GalCer. In vitro–generated NKT cells (106/mL) were cocultured with bone marrow–derived dendritic cells (105/mL) in the presence of the indicated dose of α-GalCer (100 ng/mL). ND indicates not detected. Average of triplicate and SD is shown. One representative of 3 experiments is shown.
Analysis of NKT cells derived from NKT-ES cells. (A) FACS profiles of in vitro–generated NKT cells. Cells developed from NKT-ES cells on day 20 of culture with OP9/Dll-1 (Th2-like NKT) or by the switch culture using OP9/control (Th1-like NKT) were gated on the α-GalCer/CD1d dimer+ TCRβ+ fraction and further analyzed for the expression of the indicated markers, NK1.1 versus CD3ϵ and CD4 versus CD8. (B) Surface phenotypes of in vitro–generated NKT cells. Gated fractions shown in panel A were further analyzed for the expression of the indicated markers. Shadowed profiles indicate isotype-matched control staining. (C) Proliferation and cytokine production of in vitro–generated NKT cells upon stimulation with α-GalCer. In vitro–generated NKT cells (106/mL) were cocultured with bone marrow–derived dendritic cells (105/mL) in the presence of the indicated dose of α-GalCer (100 ng/mL). ND indicates not detected. Average of triplicate and SD is shown. One representative of 3 experiments is shown.
Homogeneity of the cells with NKT-cell potential in Fr 11
To determine whether Fr 11 has a restricted developmental potential, we generated NKT-ES-V cells in which IRES-Venus was inserted downstream of the TCR Cα stop codon in NKT-ES cells (Figure 1A rows 3–4, Figure 1B) and cultured the cells at indicated time points. More than 70% of Fr 11 on day 14, but not other fractions on days 11 and 14, expressed Venus fluorescent protein (Figure 3D), confirming the data on the PCR detection of Vα14-Jα18 and Cβ transcripts (Figure 3A) and intracellular TCRβ expression (Figure 3C) in Fr 11. Thus, the cells in Fr 11 have a potential to rearrange Vα14-Jα18 segments and also to transcribe and translate their receptor genes, although they do not express their receptor on the cell surface. The frequency of cells with NKT-cell potential was estimated by the limiting dilution analysis and/or single-cell analysis to be approximately 80% on OP9/Dll-1 culture (Figure 3D), which is a similar frequency as those of Venus-positive fraction in Fr 11, whereas it was 20% on OP9/control culture. Concerning NKT-cell potential in Fr 10 as shown in Figure 2B, a small fraction (3.2%) of cells in Fr 10 was positive for Venus fluorescent protein expression on day 14 as shown in Figure 3C. Therefore, Fr 10 may contain cells with NKT-cell potential (Figure 2), but they are distinct from those in Fr 11, which do not require Notch signaling for their development (Figure 2) and express TCRβ chain (Figure 3C).
Properties of in vitro–generated NKT cells
We then analyzed properties of NKT cells generated from NKT-ES on OP9/control or OP9/Dll-1. NKT cells developed in the OP9/Dll-1 culture were Sca-1lo, CD62Lhi, IL-7Rα−, CD44lo/int, CD69lo, NKG2D−, NK1.1−, and CD24hi (Figure 4A-B), which are phenotypically similar to DP thymocytes in the adult thymus (supplemental Figure 5), and produced IL-4, but not IFN-γ (Figure 4C). The results indicate that they are of Th2-like NKT cells similar to the NK1.1− CD4+ cells in the thymus that produce mainly IL-4.15,38 Although there is no report on cytokine production of DP NKT cells, it is likely that Th2-like NKT cells developed from NKT-ES cells are similar to CD24hi DP NKT cells in the thymus. Moreover, further demonstrating their immaturity, these Th2-like NKT cells showed potent proliferative activity (Figure 4C).
Interestingly, unlike Th2-like NKT cells, the majority of NKT cells generated in the switch culture starting from day 14 on OP9/control were Th1-like NKT cells—which showed a phenotype similar to DN NKT cells in the liver; were Sca-1hi, CD62L−, IL-7Rα−, CD44hi, NKG2D+, CD24lo, NK1.1+ (Figure 4B and also see supplemental Figure 5); and produced mainly IFN-γ (Figure 4C). Moreover, Th1-like NKT cells showed little proliferative activity (Figure 4C), which is quite distinct from Th2-like NKT cells. It is noteworthy that cells in TCRlo fractions in Figure 4A are not NKT cells, because of their expression of CD11b. The yields of cells from the Notch-dependent conditions are 80 times more than the initial cell numbers, whereas those from Notch-independent conditions are only 14 times that of the 20-day culture, reflecting the proliferative activity of cells cultured under the Notch-dependent or -independent conditions as demonstrated in Figure 4C.
To test the stability of Th1-like and Th2-like NKT cells in their cytokine production, Th1-like (switch culture on day 14 with OP9/control) or Th2-like (OP9/Dll-1) NKT cells obtained at day 20 as shown in Figure 4 were further cocultured on OP9/control for an additional 5 days. Cytokine patterns of Th1-like NKT cells were not significantly changed even after they were cocultured on OP9/Dll-1 (supplemental Figure 6A), supporting the result that Th1-like NKT cells phenotypically resemble mature DN NKT cells in the liver. Interestingly, however, Th2-like NKT cells that resemble thymic NKT cells producing mainly IL-4 significantly altered their ability to produce IFN-γ and to reduce IL-4 production after coculturing with OP9/control for 5 days (supplemental Figure 6B). This finding indicates that Th2-like NKT cells become differentiated into Th1-like NKT cells that resemble mature liver NKT cells during further culturing without Notch signaling.
In vivo function of in vitro–generated NKT cells
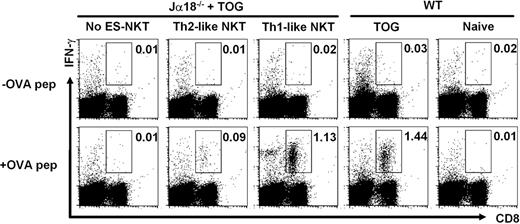
Because IFN-γ produced by NKT cells acts on NK and CD8 T cells and mediates adjuvant activity on antitumor responses, the in vivo function of Th1-like NKT cells producing mainly IFN-γ was investigated. We transferred the in vitro–generated Th1-like NKT cells into Jα18−/− mice deficient in NKT cells,7 after immunization with OVA-labeled syngeneic spleen cells from TAP−/− mice together with α-GalCer (TOG). Th2-like NKT cells generated in vitro were used as a control. The Jα18−/− recipient mice reconstituted with 2 × 106 cells of Th1-like NKT cells 1 week earlier had an increased number of OVA-specific IFN-γ–producing CD8+ T cells upon stimulation, 100-fold above the level of the mice not receiving cell transfer and comparable with that of WT mice receiving TOG treatment (Figure 5). As expected, the recipients repopulated with Th2-like NKT cells had little increase in the number of OVA-specific IFN-γ–producing CD8+ T cells. Together, the results suggest that NKT cells originated in vitro from NKT-ES cells are capable of adaptation in in vivo circumstance at least for a week and are responsible for the external stimuli available for NKT-cell activation.
In vivo adjuvant activity of in vitro–generated Th1-like NKT cells. Th1-like NKT cells were generated as shown in Figure 2C. Jα18−/− mice were reconstituted with or without (No ES-NKT) Th1-like NKT cells (2 × 106/mouse) and immunized with OVA-loaded TAP−/− spleen cells (2 × 107) and α-GalCer (2 μg; TOG immunization). One week later, spleen cells were restimulated in vitro with or without 1μM OVA peptide (257-264) as described.34 As a control, spleen cells from wild-type B6 mice or Jα18−/− mice reconstituted with Th2-like NKT cells (generated as shown in Figure 2B and that had been treated with or without TOG immunization) were stimulated in vitro with or without OVA257-264 peptide. The percentage of IFN-γ–producing CD8+ T cells stimulated with or without OVA is shown by the intracellular cytokine staining (boxed). One representative of 4 independent experiments is shown.
In vivo adjuvant activity of in vitro–generated Th1-like NKT cells. Th1-like NKT cells were generated as shown in Figure 2C. Jα18−/− mice were reconstituted with or without (No ES-NKT) Th1-like NKT cells (2 × 106/mouse) and immunized with OVA-loaded TAP−/− spleen cells (2 × 107) and α-GalCer (2 μg; TOG immunization). One week later, spleen cells were restimulated in vitro with or without 1μM OVA peptide (257-264) as described.34 As a control, spleen cells from wild-type B6 mice or Jα18−/− mice reconstituted with Th2-like NKT cells (generated as shown in Figure 2B and that had been treated with or without TOG immunization) were stimulated in vitro with or without OVA257-264 peptide. The percentage of IFN-γ–producing CD8+ T cells stimulated with or without OVA is shown by the intracellular cytokine staining (boxed). One representative of 4 independent experiments is shown.
Discussion
We generated NKT-ES cells with the idea that the prerearranged invariant Vα14-Jα18 gene would predispose the NKT-cell fate and provide advantages in investigating precursors with NKT potential and their developmental process. Using NKT-ES culture system, the cell fractions with NKT-cell potential are detected in vitro where NKT-ES cells are cultured on OP9/Dll-1. Curiously, NKT-ES–cell development was undertaken through progression that was phenotypically similar to thymic development in vivo and in vitro.37 Such a process is characterized by sequential alterations in the expression of CD44 and CD25, for example, from CD44+CD25− (DN1) to CD44+CD25+ (DN2) to CD44−CD25+ (DN3) to CD44−CD25− (DN4).23,37 In contrast, wild-type ES cells did not generate NKT cells in the same culture conditions, suggesting that the rearranged TCRVα locus characteristics for NKT cells provide an advantage for the lineage commitment, as previously detected in vivo.25
During developmental progression, NKT-ES cells generate cells with NKT-cell potential in Fr 5 displaying the phenotype on day 11 of culture with c-kitlo, RAG-1−/RAG-2+, Vα14-Jα18 transcript−, and c-kit+ (Figures 2A, 3A-B) and undergo development into α-GalCer/CD1d dimer+ NKT cells on day 20 only in the presence of Notch signaling, implying that the cells in Fr 5 are under the precommitment stage to NKT cells. On day 14 of culture, NKT-ES cells generate the cells with NKT-cell potential in Fr 11, which are positive for c-kit−, RAG-1/RAG-2, pre–TCR alpha (pTα), and Vα14-Jα18 transcripts (Figures 2A, 3A) but are still negative for Vα14 receptor expression on the cell surface (supplemental Figure 4). The cells in Fr 11 generate NKT cells either on OP9/control or OP9/Dll-1. Thus, from days 11 to 14 of culture, the lymphoid lineage commitment is progressed in NKT-ES cells by activation of gene rearrangement machinery and transcriptional regulation in TCR-associated molecules. It is likely that the cells in Fr 5 and Fr 11 represent early waves of NKT cell–biased precursors. Surprisingly, the cells in Fr 11 are quite homogenous, because more than 70% of Fr 11 expresses the Venus fluorescent protein on day 14 of culture (Figure 3C). This developmental homogeneity might be due to the expression of prerearranged invariant Vα14 receptor.
Although the cell number is limited, a small fraction (3.2%) of cells in Fr 10 seems to have NKT-cell potential, but is apparently distinct from those in Fr 11, because it requires Notch signaling for their development (Figure 2B-C) and does not express TCRβ chain (Figure 3C) at this stage. Therefore, the cells in Fr 10 may be a precursor for Fr 11, but it is difficult to further analyze their properties at present, because the number of cells with NKT potential in Fr 10 is limited. The c-kit− cells in Fr 11 are c-kitlo/−, PIR-A/B+, Flt-3+, and Thy1+ and are thus similar to the c-kitlo/− DN1 thymic progenitors or the prethymic or circulating c-kitlo/−, Thy1+, or PIR-A/B+ progenitors in adult or fetal blood circulation, which contain efficient T-lineage potential when cultured on OP9/Dll-1.39-41 The cells in Fr 11 are also positive for Flt-3 (Figure 3B) and show potent activity to generate NKT cells in vitro (Figure 2B-C). In agreement with these findings, Flt-3+ early progenitor cells are detected among DN thymocytes and show potent activity to produce DP thymocytes.42 It is also important to note that GM-CSFRα chain expression detected on the cells in Fr 11 is a characteristic property of NKT-cell precursors (Figure 3B), confirming the previous observation that GM-CSFR expression is detected in early NKT precursors.18
Concerning the role of Notch signaling, 3 important observations in the development of NKT cells are indicated: First, Notch signaling is essential for early stages of NKT-cell development (Figure 2B-C, supplemental Figure 2). Cells with NKT-cell potential are enriched in Fr 5 with c-kitlo phenotypes on day 11 of culture only when cultured on OP9/Dll-1. However, their development activity was abrogated by cultivation on OP9/control from the beginning (supplemental Figure 2) or from the switch culture on OP9/control starting at day 11 (Figure 2C). Thus, development of NKT precursor cells in vitro is dependent on the Notch signaling by day 11 of culture. These findings are in agreement with previous findings that Notch signaling is an essential factor in determining a lymphocyte's lineage fate.43
Second, NKT-ES cells undergoing the 14-day culture on OP9/Dll-1 populates c-kit− cells (Fr 11) with NKT-cell potential when recultivate on OP9/control, instead of OP9/Dll-1. It is also true that Fr 10 but not Fr 11 requires Notch signaling for the development of NKT cells. This indicates that Notch signaling during days 11 to 14 of culture is also crucial for NKT-cell development, probably from precommitment to commitment stage characterized by transcriptional activation on TCR Vα14 locus (Figure 3A).
Third, Notch signaling is not always required for differentiation of functional NKT cells (Figures 2C, 4). The NKT-ES cells differentiate finally into NKT cells, producing mainly IL-4 but not IFN-γ (Th2-like NKT cells) in a Notch-dependent fashion. On the other hand, the same NKT-ES cells, when developed in the switch culture starting at day 14 without Notch signaling, give rise to NKT cells that resemble liver NKT cells (Th1-like NKT cells) producing IFN-γ and small amounts of IL-4 and exhibiting strong adjuvant effects in vivo (Figures 4C, 5). Therefore, it is clear that functional maturation of NKT cells occurs in the absence of Notch signaling, a finding that is in line with a previous report in which γδT cells were shown to be less sensitive to the requirement for Notch signaling.44 Thus, sustained Notch signaling is critical for the initial step of NKT-cell development, although Notch signaling may have negative effects on functional maturation of NKT cells.
The precise mechanisms by which Notch signaling determines function (Th1-like or Th2-like) of NKT cells remain unclear. However, Notch signaling is required for Th2 differentiation via GATA-binding protein 3 (GATA3), because an upstream GATA3 promoter is a direct target for Notch signaling, and also because only Th2 responses are blocked in mice deficient in the Notch-associated DNA-binding factor, RBP-J.45,46 Conversely, in the absence of GATA3, Notch signaling leads to Th1, not Th2, differentiation. Analogous to these Notch-mediated control mechanisms of Th1/Th2 differentiation, it is likely that Notch signaling is a critical element determining Th2 function characterized by IL-4 production. Because Notch ligands are highly expressed on thymic epithelial cells, NKT precursors might acquire Th2-like potential and then undergo maturation in the absence of Notch signaling.
In the developmental process of mature NKT cells diverged from DP thymocytes, 3 stages are described: first a CD44lo NK1.1− stage, then a CD44hi NK1.1− stage, and finally a CD44hi NK1.1+ stage.15,38 These developmental stages are strongly associated with the defined functional changes. The CD44lo NK1.1− cells are exclusively IL-4 producers, whereas CD44hi NK1.1− cells produce both IL-4 and IFN-γ and CD44hi NK1.1+ cells produce more IFN-γ than IL-4.15,38 The in vitro–generated Th2-like NKT cells from NKT-ES cells are phenotypically similar to the thymic NKT cells at the CD44lo NK1.1− stage, whereas Th1-like NKT cells derived from NKT-ES cells are likely to be DN NKT cells in the liver.
Finally, Th1-like NKT cells with their adjuvant activity are useful for protective immunity, whereas Th2-like NKT cells might be used to prevent autoimmune diseases and maintenance of transplantation tolerance. Thus, the availability of NKT cells with desired functions in vitro offers a powerful new approach for the establishment of NKT-cell therapy.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank P. D. Burrows and T. Takemori for critical reading and N. Takeuchi for secretarial assistance.
This study was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan (M.T. and H.W.).
Authorship
Contribution: H.W. and A.R. performed research; N.H., Y.N., S.S., E.S., N.D., T.T., S.-i.F., K.S., and K. Masuda contributed to some experiments; K. Mori, H. Kawamoto, and H. Koseki helped edit the paper; and M.T. designed research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Masaru Taniguchi, RIKEN Research Center for Allergy and Immunology, 1-7-22, Suehiro-cho, Tsurumi, Yokohama, 230-0045, Japan; e-mail: taniguti@rcai.riken.jp.
References
Author notes
*H.W., A.R., and N.H. shared equally in the preparation of this paper.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal