Abstract
The efficacy of therapeutic angiogenesis for revascularization in ischemia using genes, proteins, and cells has been established. For further improvement, processes allowing enlargement of the luminal cavity to facilitate efficient blood flow need to be facilitated. Recently, we found that expression of APJ and its specific ligand, apelin, is seen in endothelial cells when angiogenesis is taking place during embryogenesis. Apelin-deficient mice are viable but have narrow intersomitic vessels during embryogenesis and narrow blood vessels in the trachea and skin after birth. Apelin induces the formation of larger cords of endothelial cells, mainly mediated by cell-cell aggregation, resulting in the generation of larger blood vessels. Here we report that transgenic overexpression of apelin in keratinocytes induces enlarged but not leaky blood vessels in the dermis. In the hind limb ischemia model, apelin together with vascular endothelial growth factor (VEGF) effectively induced functional vessels larger than with VEGF alone. Endogenous apelin is required for the suppression of VEGF-, histamine-, or inflammation-induced vascular hyperpermeability. Apelin inhibited the down-modulation of vascular endothelial-cadherin by VEGF, resulting in suppression of hyperpermeability. Our results suggest apelin efficacy for therapeutic angiogenesis.
Introduction
Vascular stenosis in large arteries caused by atherosclerosis, inflammatory processes, and other events can lead to ischemia in different tissues and organs. Ischemia compromises microvascular function by damaging vascular endothelial cells (ECs), resulting in the reduction of functional capillaries and progression of ischemic ulceration and gangrene. Therefore, therapeutic angiogenesis is essential for restoration of blood flow to ischemic tissues and organs. Local administration of genes encoding proangiogenic factors, such as vascular endothelial growth factor-A (VEGF), basic fibroblast growth factor (bFGF, FGF2), and hepatocyte growth factor, has been undertaken to manage patients with severe myocardial ischemia1,2 or critical limb ischemia3,4 refractory to conventional therapies. It has been reported that therapy with such genes enhanced collateral circulation by promoting angiogenesis in patients with critical limb ischemia disease.5,6 In particular, gene therapy using the FGF1 expression plasmid NV1FGF resulted in a good outcome in patients with critical limb ischemia in phase 1 and 2 trials.7 However, it remains uncertain whether the administration of a single proangiogenic factor is sufficient to promote the formation of mature vessels that can provide adequate blood flow to ischemic lesions. Indeed, single gene administration of VEGF, bFGF, or hepatocyte growth factor failed to demonstrate significant benefits in early phase 1 and 2 human clinical trials.8-10
In the process of angiogenesis, proliferation and migration of ECs are required; subsequently, EC-to-EC sealing is induced by tight junction formation, and finally newly developed blood vessels are stabilized by adhesion to mural cells, such as pericytes or smooth muscle cells. These processes are not regulated by a single molecule but coordinately by several angiogenic factors.11,12 Therefore, excessive amounts of a single proangiogenic factor, ignoring the balance between proangiogenic and antiangiogenic factors, may induce disorganized blood vessels as observed in the tumor environment. Indeed, some reports suggest that excessive VEGF expression induces pathologic and immature vessel formation consisting of nascent capillaries, resulting in plasma leakage and tissue edema.13-15 Therefore, it is suggested that therapeutic angiogenesis should be controlled by angiogenesis as observed in vivo and especially that regulation of stabilization and maturation processes is required for induction of functional blood vessel formation.
Combination gene therapy with several growth factors is a rational approach to creating more stable vessels for functional improvement in ischemic tissue. Among such factors, angiopoietin-1 (Ang-1), a ligand for Tie2 on ECs, plays a critical role in promoting adhesion between mural cells and ECs, resulting in stabilization and enlargement of blood vessels.16-18 Coadministration of VEGF and Ang-1 modified angiogenesis and reduced vascular permeability in the skin of transgenic (Tg) mice and in ischemic hind limb models.19-21 These combination gene therapies are currently being examined in animal models in preparation for translation to clinical human therapy.22 Thus, identification and use of maturation factors are required to achieve optimal therapeutic angiogenesis.
Apelin has been identified as the endogenous ligand of the G protein–coupled receptor APJ.23 Apelin and APJ mediate a wide range of physiologic actions, including angiogenesis,24-27 heart contractility and blood pressure regulation,28 appetite and drinking behavior,29 immune responses,30 and other effects.31,32 Apelin and APJ are expressed on ECs of the newly developing blood vessels during angiogenesis,24 and it has been reported that apelin expression is induced by hypoxia in ECs.26 In vitro analysis revealed that apelin stimulates proliferation, migration, and tube formation of ECs.33,34 Recently, we reported that apelin-deficient mice are viable but have narrow intersomitic vessels during embryogenesis and narrow blood vessels in the trachea and skin after birth. Apelin induces larger cords of ECs mainly mediated by cell-cell aggregation, resulting in formation of enlarged blood vessels.24 Apelin expression is induced by the stimulation of Tie2 on ECs, and we found that Ang-1–mediated vascular enlargement35 was abrogated in apelin-deficient mice.24 Moreover, it has been reported that apelin deficiency significantly impaired retinal vascularization in the early postnatal period.25 Taken together, these data support the notion that the apelin/APJ pathway is important for vascular formation, especially for regulating caliber size of blood vessels to facilitate lumen enlargement.18,24
In the present study, we generated Tg mice expressing apelin in the epidermis under the transcriptional control of the K14 promoter and observed the in vivo effects of apelin on blood vessel caliber size regulation. Moreover, we also studied the effects of apelin induction together with VEGF on functional recovery from ischemia in the mouse hind limb ischemia model. Furthermore, we analyzed the precise mechanism of how apelin induces nonleaky blood vessels associated with EC-to-EC junction fidelity mediated by vascular endothelial (VE)–cadherin.
Methods
Animals
ICR and C57BL/6 mice were purchased from Japan SLC. The pK14-apelin-pA plasmid was generated by inserting the coding region of mouse apelin cDNA into the pK14-pA plasmid.36 We generated K14-apelin Tg mice according to standard methods.37 We identified Tg offspring by polymerase chain reaction (PCR) of tail genomic DNA using forward (5′-GCC TGT GGG TGA TGA AAG CC-3′) and reverse (5′-GGT CCA GTC CTC GAA GTT CT-3′) primers. Three independent K14-apelin TG lines were backcrossed with wild-type (WT) C57BL/6 mice. Generation of apelin−/− mice was described previously.24 Animals were housed in environmentally controlled rooms of the animal experimentation facility at Osaka University. All experiments were carried out under the guidelines of Osaka University Committee for animal and recombinant DNA experiments and were approved by the Osaka University Institutional Review Board.
Quantitative real-time PCR analysis
Total RNA was extracted from cells and tissues using the RNeasy Plus Mini kits (QIAGEN) and transcribed into cDNA using Exscript RT Reagent kits (Takara) according to the manufacturer's protocol. Primers for APJ, apelin, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were previously described.24 Real-time PCR analysis was performed by Platinum SYBR Green qPCR SuperMix-UDG (Invitrogen). The levels of PCR products were monitored with Mx3000P QPCR System (Stratagene). The baseline and threshold were adjusted according to the manufacturer's instructions. The relative abundance of transcripts was normalized using either the expression level of GAPDH mRNA or CD31 mRNA.
Western blot analysis
The human VE-cadherin expression vector was a kind gift of Dr Fukuhara (National Cardiovascular Center, Osaka, Japan). Lipofectamine Plus reagent (Invitrogen) was used to transfect cells with this plasmid. Cell surface and cytoplasmic VE-cadherin expression by human umbilical vein endothelial cells (HUVECs) was analyzed using a Mem-Per Reagent kit (Thermo) according to the manufacturer's protocol. Immunoblotting was performed to detect expression of VE-cadherin, N-cadherin, apelin, VEGF, and GAPDH. Total cell lysates (2 μg total protein) were heated for 2 minutes at 95°C and then loaded onto sodium dodecyl sulfate-polyacrylamide gels. Proteins were electrophoretically transferred onto polyvinylidene difluoride membranes, blocked with 5% nonfat dry milk, and subsequently incubated with anti–VE-cadherin, anti–N-cadherin (BD Bioscience), anti-apelin27 (a gift from Takeda Pharmaceutical Company), anti-VEGF (Santa Cruz Biotechnology), or anti-GAPDH antibodies (Abs; Chemicon) according to the manufacturer's protocol. Proteins were detected with horseradish peroxidase-conjugated anti–mouse, anti–rabbit (Dako North America), or anti–rat secondary Ab (BioSource International) and ECL reagents (GE Healthcare). In all experiments, the blots were scanned with an imaging densitometer LAS-3000 mini (Fujifilm). Predetermined molecular weight standards were used as markers. Protein concentration was measured by the protein assay dye reagent (Bio-Rad) with bovine serum albumin as a standard.
Immunostaining and lectin staining
Sections from back skin and ears of K14-apelin Tg and WT mice were stained with anti–mouse CD31 antibody (BD Biosciences PharMingen), anti–mouse APJ antibody,24 or anti–mouse apelin antibody.24 The procedure for tissue preparation and staining was as previously reported.38 HUVECs (Kurabo) were cultured in Humedia EG2 as described.24 HUVECs were grown to confluence, serum starved, and stimulated with various factors. The cells were then fixed with 4% paraformaldehyde and permeabilized by 0.1% Triton X-100, labeled with anti–VE-cadherin antibody (BD Biosciences) and anti–p120-catenin antibody (Cosmobio), and visualized with Alexa Fluor 488-conjugated goat ant–irat IgG and Alexa Fluor 546-conjugated goat anti–mouse IgG (Invitrogen). F-actin was stained with tetramethylrhodamine isothiocyanate-conjugated Phalloidin (Sigma-Aldrich). Nuclear staining was performed using Hoechst 33258 (Sigma-Aldrich) or TOPRO3 (Invitrogen). Internalization analysis of VE-cadherin was performed as previously reported.39 Briefly, cells were incubated in Dulbecco modified Eagle medium with anti–VE-cadherin antibody at 4°C for 1 hour. Antibody uptake was induced for 30 minutes at 37°C in serum-free medium or in the presence of VEGF and/or apelin. Cells were either fixed or subjected to a mild acid wash (2mM phosphate-buffered saline [PBS]-glycine, pH 2.0, 15 minutes) to remove plasma membrane-bound antibodies. Lectin staining was performed as previously reported40 using fluorescein isothiocyanate (FITC)–isolectin (Vector Laboratories). Samples were visualized using conventional microscopy (with a DM5500B equipped with HCX PL FLVOTAR 5/0.15 and HCX PL FLVOTAR 10×/0.15 dry objective lenses; Leica) or confocal microscopy (TCS/SP5 equipped with HC PLAN APO 20×/0.70 and HCXPL APO 40×/1.25-0.75 oil objective lenses; Leica). Images were acquired with a DFC 500 digital camera (Leica) and processed with the Leica application suite (Leica) and Adobe Photoshop CS3 software (Adobe). All images shown are representative of 3 to 5 independent experiments.
Plasma extravasation
To determine vascular permeability, a Miles assay was performed in mice as previously reported.41 Mice (ICR, C57 BL/6, K14 apelin Tg, or apelin−/−) were anesthetized and shaved. After 2 to 3 days, mice were anesthetized again and intravenously injected with 150 μL of 1% Evans blue dye. After 15 minutes, intradermal injection of factors was performed as follows: 15 μL of VEGF (PeproTech), histamine (Wako), recombinant apelin peptide (Bachem AG) at different concentrations, or PBS as a negative control. The dye was eluted from the dissected samples with formamide at 56°C, and the optical density was measured by spectrophotometry (Biotrak II; GE Healthcare) at 620 nm.
Flow cytometric analysis
Hind limb muscle tissue cells were pretreated with Fc-Blocker (BD Biosciences PharMingen) and stained with FITC-conjugated anti-CD45 monoclonal antibody and phycoerythrin-conjugated anti-CD31 monoclonal antibody (BD Biosciences PharMingen). Procedures for cell preparation and staining were as previously reported.40 The stained cells were analyzed by FACSCalibur (BD Biosciences) and sorted using a JSAN flow cytometer (Bay Bioscience). Dead cells were excluded from the analyses using the 2-dimensional profile of forward versus side scatter.
Hind limb ischemia model and injection of plasmids
Seven-week-old male apelin KO or C57BL/6 mice underwent surgery to induce unilateral hind limb ischemia as previously described.40 Briefly, after anesthesia by isoflurane inhalation, the left femoral artery was exposed under a stereomicroscope. The artery was then ligated both proximally and distally using 6-0 silk sutures, and the ligated vessels were resected between the ligatures without damaging the femoral nerve. Sham operations involved skin incision without femoral artery ligation. The mice were then injected with pCAG-LacZ, pCAG-VEGF, and/or pCAG-apelin plasmid using GenomOne-Neo transfection reagent (Ishihara Sangyo) into the quadriceps muscle of the ischemic limb according to the manufacturer's protocol. Rate of blood flow was measured 2 weeks after surgery by a laser Doppler blood flow meter (Moor LDI2-IR; Moor Instruments) in both the ischemic and nonischemic hind limbs of the same animal. The blood flow values were expressed as the ratio of ischemic to nonischemic hind limb perfusion. Capillary number and size in the gastrocnemius muscle were assessed by immunohistochemistry as described in “Immunostaining and lectin staining” using rat anti–mouse CD31 antibody (BD Biosciences PharMingen).
Statistical analysis
Data are mean plus or minus SD and analyzed by repeated-measures 2-way analysis of variance or Student t test using Statview software (Abacus Concepts). A probability value of less than .05 was considered statistically significant.
Results
Tg overexpression of apelin induces larger blood vessels
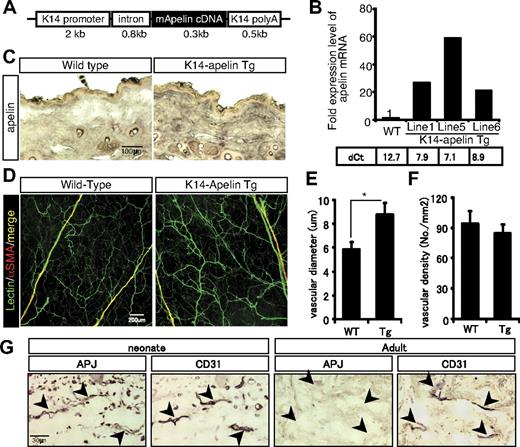
To ascertain the functional significance of apelin for blood vessel formation in vivo, we generated Tg mice on the C57BL/6 background (K14-apelin Tg mice) expressing apelin in basal epidermal keratinocytes under the transcriptional control of the K14 promoter (Figure 1A). Real-time PCR analysis of skin mRNA obtained from WT and from K14-apelin mice 3 days after birth revealed that the transgene was incorporated into 8 founder mice (data not shown). Three independent Tg mouse lines (#1 #5, and #6) were chosen for analysis because of their high level expression of apelin mRNA (Figure 1B).
Transgenic overexpression of apelin induces larger blood vessels. (A) Schematic representation of the K14-apelin expression cassette. (B) Quantitative real-time PCR analysis of apelin mRNA in the ear skin from 3-day-old WT mice and animals from 3 different founder lines of K14-apelin Tg mice. Threshold values for target genes normalized against the level of GAPDH (dCt) are shown under the graph. (C) Immunohistochemical staining of apelin expression in the dorsal skin sections from 6-week-old WT or K14-apelin Tg mice. Positive reactions are revealed by purple coloration. (D) Comparison of blood vessels in lectin-stained whole mounts of ear skin from 8-week-old WT or K14-apelin Tg mice. ECs were stained by perfusion of FITC-conjugated isolectin (green), and mural cells were stained with anti-αsmooth muscle actin (α-SMA) antibody (red). (E-F) Quantitative evaluation of the vascular diameter (E) and vascular density (F) of capillaries in the dermis from WT and K14-apelin Tg mice. Data were obtained by counting 5 random fields from 5 different mice (total 25 fields). *P < .01. (G) Immunohistochemical staining of APJ expression in the dorsal skin serial sections from 2-day-old (neonate) or 8-week-old (adult) mice. Blood vessels were detected by immunohistochemical staining with anti-CD31 Ab. Positive reactions are visualized as a purple coloration.
Transgenic overexpression of apelin induces larger blood vessels. (A) Schematic representation of the K14-apelin expression cassette. (B) Quantitative real-time PCR analysis of apelin mRNA in the ear skin from 3-day-old WT mice and animals from 3 different founder lines of K14-apelin Tg mice. Threshold values for target genes normalized against the level of GAPDH (dCt) are shown under the graph. (C) Immunohistochemical staining of apelin expression in the dorsal skin sections from 6-week-old WT or K14-apelin Tg mice. Positive reactions are revealed by purple coloration. (D) Comparison of blood vessels in lectin-stained whole mounts of ear skin from 8-week-old WT or K14-apelin Tg mice. ECs were stained by perfusion of FITC-conjugated isolectin (green), and mural cells were stained with anti-αsmooth muscle actin (α-SMA) antibody (red). (E-F) Quantitative evaluation of the vascular diameter (E) and vascular density (F) of capillaries in the dermis from WT and K14-apelin Tg mice. Data were obtained by counting 5 random fields from 5 different mice (total 25 fields). *P < .01. (G) Immunohistochemical staining of APJ expression in the dorsal skin serial sections from 2-day-old (neonate) or 8-week-old (adult) mice. Blood vessels were detected by immunohistochemical staining with anti-CD31 Ab. Positive reactions are visualized as a purple coloration.
No major phenotypic differences were observed between these lines, and Tg offspring were born in normal Mendelian ratios. Induced apelin mRNA was translated into protein and expressed specifically in the dermal layer of dorsal skin in K14-apelin Tg mice (Figure 1C). Lectin staining revealed that capillaries, but not arteriola and venula in the dermis, were larger than in WT mice (Figure 1D-E). However, vascular density was similar (Figure 1F). Ang-1 overexpression in the skin induces formation of large blood vessels without affecting the thickness of ear skin.17 The same was true for the K14-apelin Tg mice (data not shown).
To confirm apelin receptor APJ expression, we compared APJ levels in ECs from skin in neonates and adults. APJ was highly expressed in vascular ECs of neonatal skin but was barely detectable in adult skin (Figure 1G). This finding is consistent with our previous report that APJ expression is observed in ECs primarily when angiogenesis is taking place.27 These results indicate that apelin can induce nonleaky larger blood vessels in vivo.
Endogenous apelin is required for recovery of hind limb perfusion after induction of ischemia
We previously reported the temporal expression of APJ in ECs during angiogenesis in embryos.24 To determine whether APJ is expressed in a cell type-specific manner in ECs under hypoxia, occlusion of the femoral artery was used to induce ischemia in collateral vessels of the hind limb. As with VEGF induction, we found that expression of APJ as well as apelin mRNA was significantly increased by hypoxia in this model (supplemental Figure 1A-C, available on the Blood website; see the Supplemental Materials link at the top of the online article). APJ induction was observed beginning at day 2 after ischemia and then gradually attenuated. Apelin expression was slightly slower than APJ expression (supplemental Figure 1A-B). These expression profiles are consistent with our previous finding that VEGF induces APJ expression on ECs during the initial step of angiogenesis and that Ang-1 subsequently induces apelin expression on blood vessel maturation.24 Accumulation of apelin mRNA was increased when ECs were stimulated with Ang-1 under hypoxic conditions compared with normoxic conditions (supplemental Figure 1D). This might be effected by Tie2 up-regulation in ECs under hypoxia, as previously reported.42
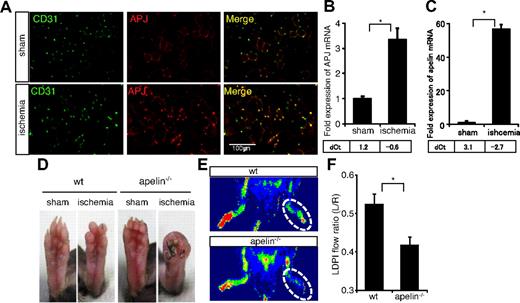
To determine whether APJ expression in the ischemic muscle is by ECs, CD31 expression was also assessed (Figure 2A; supplemental Figure 1E). In the steady state (sham-operated muscle), APJ expression was weak in CD31+ ECs; however, consistent with our previous findings that ECs start to express APJ on stimulation with VEGF,24 we observed that most CD31+ ECs of newly developing vessels in the hypoxic region strongly expressed APJ after induction of ischemia. We sorted CD31+CD45−ECs or CD31−non-ECs from ischemic muscle and confirmed specific expression of APJ and apelin in ECs under hypoxia (Figure 2B-C). These results therefore suggest the involvement of the apelin/APJ system in collateral vessel formation during the process of recovery from ischemic states.
Requirement for endogenous apelin for recovery of hind limb perfusion after induction of ischemia. (A) Immunohistochemical staining of muscle sections from sham-operated (sham) or ischemic (ischemia) hind limb with anti-CD31 (green) and anti-APJ (red) antibodies. (B-C) Quantitative real-time PCR analysis of APJ (B) or apelin (C) mRNA expression in CD31+CD45− ECs or CD31−non-ECs derived from muscle of sham-operated (sham) or ischemic (ischemia) hind limb. Levels were normalized against the level of expression of CD31 as an EC marker. Threshold values for target genes normalized against the level of CD31 (dCt) are shown below the graph. (D) Gross appearance of foot pad from sham-operated (sham) or ischemic WT and apelin−/− mice 14 days after resection of the femoral artery. (E) Representative laser Doppler images of mouse hind limbs from WT or apelin−/− mice 14 days after surgery. Red represents greater flow; and blue, less flow. (F) Quantification of laser Doppler-monitored blood flow measurements. Data are from ratio of ischemic right leg versus nonischemic left leg in mice (n > 10). *P < .01.
Requirement for endogenous apelin for recovery of hind limb perfusion after induction of ischemia. (A) Immunohistochemical staining of muscle sections from sham-operated (sham) or ischemic (ischemia) hind limb with anti-CD31 (green) and anti-APJ (red) antibodies. (B-C) Quantitative real-time PCR analysis of APJ (B) or apelin (C) mRNA expression in CD31+CD45− ECs or CD31−non-ECs derived from muscle of sham-operated (sham) or ischemic (ischemia) hind limb. Levels were normalized against the level of expression of CD31 as an EC marker. Threshold values for target genes normalized against the level of CD31 (dCt) are shown below the graph. (D) Gross appearance of foot pad from sham-operated (sham) or ischemic WT and apelin−/− mice 14 days after resection of the femoral artery. (E) Representative laser Doppler images of mouse hind limbs from WT or apelin−/− mice 14 days after surgery. Red represents greater flow; and blue, less flow. (F) Quantification of laser Doppler-monitored blood flow measurements. Data are from ratio of ischemic right leg versus nonischemic left leg in mice (n > 10). *P < .01.
To determine the requirement for endogenous apelin in recovery from the ischemic state, hind limb ischemia was induced in apelin-deficient mice (apelin−/− mice) and WT mice. After 2 weeks, severe necrosis of the toes was observed in apelin−/− but not WT mice (Figure 2D). Delayed revascularization in the apelin−/− mice was assessed by laser Doppler-monitored blood flow measurements (Figure 2E-F). Thus, we found that APJ expression is induced after ischemic treatment and endogenous apelin is required for functional recovery.
To exclude the possibility that apelin deficiency leads to attenuation of hematopoietic cell recruitment in the ischemic hind limb muscle, which then enhances angiogenesis, we investigated the frequency of CD45+ and APJ+ hematopoietic cells there. However, no major differences in the number of recruited CD45+ hematopoietic cells between WT and apelin−/− mice were found, and we failed to detect APJ+ cells among the CD45+ hematopoietic cells in either (data not shown). This suggests a direct effect of apelin on ECs.
Apelin gene transfer promotes development of larger vessels in the hind limb ischemia model
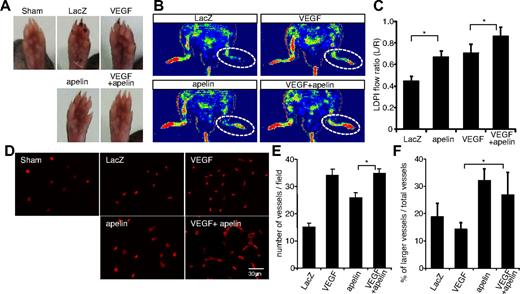
Although apelin in WT mice is up-regulated in ECs of ischemic muscle (Figure 2C), additional apelin induction may improve blood vessel formation. Therefore, we next evaluated the effect of apelin gene transfer in this hind limb ischemia model and compared apelin induction alone with both apelin and VEGF together. Mouse apelin, human VEGF-165 (VEGF), and β-galactosidase expression plasmids were constructed (supplemental Figure 2A). Protein expression was confirmed by Western blotting and immunohistochemical analysis 24 hours after gene transfer to hind limb muscle (supplemental Figure 2B-C). Necrotic toe was observed in mice receiving control LacZ gene therapy but was reduced by VEGF or apelin gene transfer. Moreover, such necrosis was essentially undetectable in mice receiving both VEGF and apelin gene therapy (Figure 3A). Efficiency of revascularization was assessed by laser Doppler-monitored blood flow measurements (Figure 3B-C). Gene therapy with either apelin or VEGF alone improved blood flow, but a combination of both was most effective.
Apelin gene transfer promotes development of larger vessels in the hind limb ischemia model. (A) Gross appearance of foot pad from sham-operated mice (sham) or ischemic mice injected with genes as indicated 14 days after resection of the femoral artery. (B) Representative laser Doppler images of a mouse hind limb 14 days after surgery and gene transfer as indicated. Red represents greater flow; and blue, less flow. (C) Quantification of laser Doppler-monitored blood flow measurements. Data are from ratio of ischemic right leg versus nonischemic left leg mice injected with genes as indicated (n > 10). *P < .01. (D) CD31 staining of quadriceps muscle from mice 14 days after surgery and gene transfer as indicated. Muscle from sham-operated (sham) mice was used for comparison. (E-F) Quantitative evaluation of the vessel number (E) and the percentage of enlarged blood vessels more than 30 μm relative to the total number of blood vessels (F) in mice 14 days after surgery and gene transfer as indicated. *P < .01.
Apelin gene transfer promotes development of larger vessels in the hind limb ischemia model. (A) Gross appearance of foot pad from sham-operated mice (sham) or ischemic mice injected with genes as indicated 14 days after resection of the femoral artery. (B) Representative laser Doppler images of a mouse hind limb 14 days after surgery and gene transfer as indicated. Red represents greater flow; and blue, less flow. (C) Quantification of laser Doppler-monitored blood flow measurements. Data are from ratio of ischemic right leg versus nonischemic left leg mice injected with genes as indicated (n > 10). *P < .01. (D) CD31 staining of quadriceps muscle from mice 14 days after surgery and gene transfer as indicated. Muscle from sham-operated (sham) mice was used for comparison. (E-F) Quantitative evaluation of the vessel number (E) and the percentage of enlarged blood vessels more than 30 μm relative to the total number of blood vessels (F) in mice 14 days after surgery and gene transfer as indicated. *P < .01.
Immunohistochemical analysis of hind limb muscle 2 weeks after gene transfer revealed that VEGF alone effectively increased the number of blood vessels (Figure 3D-F), whereas apelin induced fewer but larger vessels than VEGF. Although the proportion of enlarged among total blood vessels was slightly reduced, the combination of VEGF and apelin still resulted in increased numbers of larger vessels than VEGF alone (Figure 3D-F).
Apelin suppresses VEGF-induced vascular edema
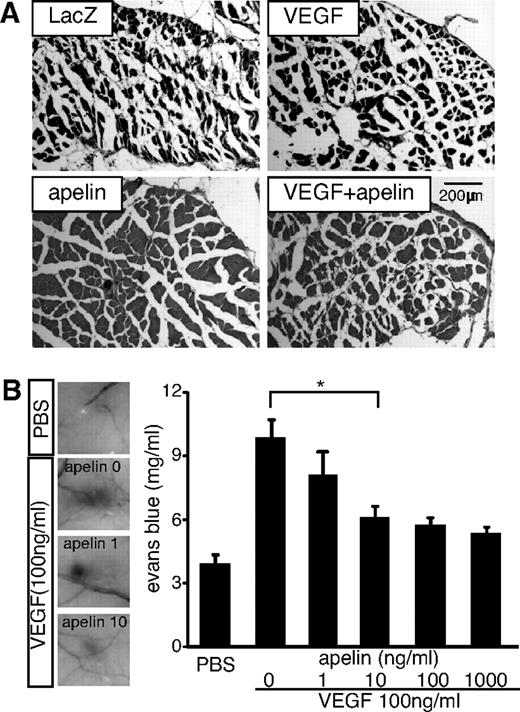
Vascular leakage resulting in tissue edema is a major problem in therapeutic angiogenesis.13,43 It has been reported that Ang-1 induces enlarged blood vessels and inhibits VEGF-mediated hyperpermeability.17 We found that apelin expression is induced in ECs by Ang-1.24 Therefore, we asked whether apelin also inhibits VEGF-mediated leakiness of blood vessels. In hind limb muscle of VEGF-treated mice, abundant edematous free spaces in the gastrocnemius muscles were observed, resulting in contraction of muscle fibers. In contrast, apelin-treated mice lacked such edema; moreover, VEGF-mediated edema was suppressed in mice receiving apelin/VEGF double-gene therapy (Figure 4A). We measured the circumference of the hind limbs, which reflects the degree of angioedema, and found that femoral artery occlusion itself resulted in swollen legs. Apelin also rapidly reduced this and inhibited VEGF-mediated swelling (supplemental Figure 3).
Apelin suppresses VEGF-induced vascular edema. (A) Histologic analysis of hind limb muscles 14 days after resection of the femoral artery and gene therapy as indicated. (B) Miles assay using VEGF and apelin. Left-hand panels: Representative images of the vascular leakage induced by VEGF in the presence or absence of apelin (1 or 10 ng/mL). PBS was used as a negative control. Right-hand panel: Evans blue dye content eluted from dissected skin (mean ± SEM; n = 10). *P < .01.
Apelin suppresses VEGF-induced vascular edema. (A) Histologic analysis of hind limb muscles 14 days after resection of the femoral artery and gene therapy as indicated. (B) Miles assay using VEGF and apelin. Left-hand panels: Representative images of the vascular leakage induced by VEGF in the presence or absence of apelin (1 or 10 ng/mL). PBS was used as a negative control. Right-hand panel: Evans blue dye content eluted from dissected skin (mean ± SEM; n = 10). *P < .01.
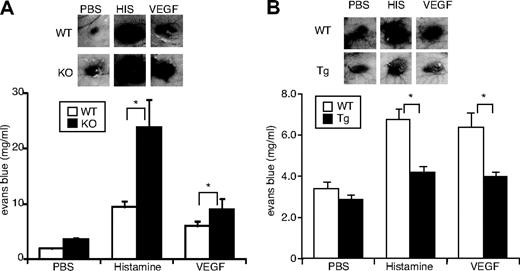
To study the role of apelin in vascular permeability, we applied a modified Miles vascular hyperpermeability assay in vivo. As shown in Figure 4B, VEGF-mediated hyperpermeability was suppressed by apelin in a dose-dependent manner. Similarly, histamine-mediated vascular permeability was also suppressed by apelin (supplemental Figure 4). To ascertain the requirement for endogenous apelin, the Miles assay was performed using apelin−/− mice. Vascular hyperpermeability mediated by VEGF or histamine was increased by the lack of apelin (Figure 5A). In contrast, overexpression of apelin inhibited vascular leakage caused by VEGF or histamine in K14-apelin Tg mice (Figure 5B). Furthermore, plasma leakage caused by topical application of mustard oil, an inflammatory agent, was enhanced in apelin−/− mice and suppressed in K14-apelin Tg mice compared with WT mice (supplemental Figure 5). These data show that apelin inhibits vascular leakage caused by several different agents.
Requirement for endogenous apelin for inhibition of VEGF- or histamine-induced vascular edema. (A-B) Vessel permeability was assessed by the Miles assay. Apelin-deficient (KO) and WT mice (A) or K14-apelin Tg and WT mice (B) were injected with 15 μL of PBS in the presence or absence of rhVEGF165 (100 ng/mL) or histamine (100μM) intradermally. Thirty minutes after injection, the inner side of the dorsal skin was inspected. Top panels: Representative images of the vascular leakage. Bottom panels: Quantification of extravasated Evans blue dye content eluted from dissected skin (mean ± SEM; n = 10). *P < .05.
Requirement for endogenous apelin for inhibition of VEGF- or histamine-induced vascular edema. (A-B) Vessel permeability was assessed by the Miles assay. Apelin-deficient (KO) and WT mice (A) or K14-apelin Tg and WT mice (B) were injected with 15 μL of PBS in the presence or absence of rhVEGF165 (100 ng/mL) or histamine (100μM) intradermally. Thirty minutes after injection, the inner side of the dorsal skin was inspected. Top panels: Representative images of the vascular leakage. Bottom panels: Quantification of extravasated Evans blue dye content eluted from dissected skin (mean ± SEM; n = 10). *P < .05.
Dysfunction of lymphatic vessels and hemodynamic disorder may also cause tissue edema. To determine whether apelin directly affects vascular hyperpermeability, we tested apelin inhibition of VEGF-mediated leakage using EC monolayers in vitro. We confirmed that apelin stimulation abrogates FITC-dextran leakage through EC monolayers exposed to VEGF (supplemental Figure 6).
Apelin inhibits internalization of VE-cadherin
To examine the mechanism of inhibition of vascular leakage, we used HUVECs to investigate the role of apelin in the formation of intracellular gap and cellular junctions in vitro. It has been reported that VE-cadherin–forming EC-EC junctions are lost and gaps between ECs are induced on stimulation with permeability factors.39,44 VEGF stimulation induced stress fibers and destroyed the VE-cadherin–mediated cell-cell junction, resulting in gap formation in HUVECs (Figure 6A). Apelin alone did not affect cell-cell junctions, but the effect of VEGF on gap formation was abolished by apelin pretreatment, and overexpression of VE-cadherin on HUVECs mimics the effect of apelin (supplemental Figure 7A-B). Similar suppressive effects of apelin on gap formation were observed using histamine (supplemental Figure 8A-C). Time-lapse imaging of cultured ECs revealed that histamine and VEGF stimulation induced dynamic cell motility and large intercellular gap formation and showed that this was completely suppressed by pretreatment with apelin (supplemental Videos 1-5). Gap formation suggests disruption of EC-EC barrier function by VEGF or histamine. Indeed, histamine rendered EC monolayers permeable to FITC-dextran. This leakage was almost completely abrogated by stimulation with apelin (supplemental Figure 8D).
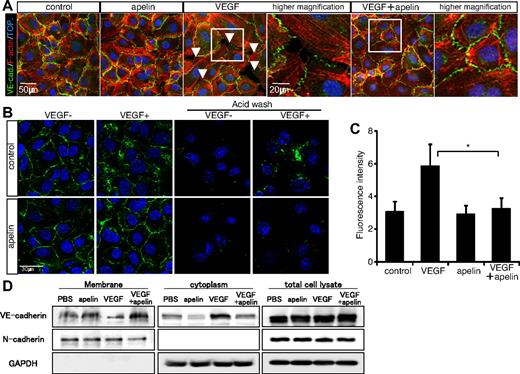
Apelin inhibits VEGF-mediated hyperpermeability. (A) Effect of apelin on VE-cadherin–mediated endothelial junctions. VE-cadherin expression was studied in HUVEC monolayers. HUVECs cultured with or without pretreatment with apelin for 10 minutes were stimulated with VEGF (20 ng/mL) for 10 minutes. F-actin (red) and VE-cadherin (VE-cad.; green) staining is shown. Arrowheads indicate the gaps between adjacent ECs and lack of VE-cadherin. Nuclei were labeled with TOPRO-3 (TOP.; blue). (B) Suppression of VEGF-mediated VE-cadherin internalization by apelin. HUVEC cell surface VE-cadherin labeled by anti–VE-cadherin antibody and stimulated with VEGF (20 ng/mL) in the presence (apelin) or absence (control) of apelin (100 ng/mL). VE-cadherin internalization was visualized in fixed cells using secondary fluorescent antibodies (green). To confirm the internalization of VE-cadherin, cell surface bound VE-cadherin antibodies were removed by a mild acid wash before fixation (right 2 lines of images). Nuclei were labeled with TOPRO-3 (blue). (C) The internalization of endogenous VE-cadherin (in the right 2 lines of panel B) was quantified by the fluorescence intensity of secondary antibodies per cell. *P < .01. (D) Western blot analysis of cell surface and cytoplasmic VE-cadherin protein on HUVECs that had been stimulated with VEGF (20 ng/mL) and/or apelin (100 ng/mL) for 20 minutes. The purity of cytoplasmic protein was confirmed by the lack of expression of the cell surface protein N-cadherin. GAPDH expression was analyzed as a control for an unrelated intracellular protein.
Apelin inhibits VEGF-mediated hyperpermeability. (A) Effect of apelin on VE-cadherin–mediated endothelial junctions. VE-cadherin expression was studied in HUVEC monolayers. HUVECs cultured with or without pretreatment with apelin for 10 minutes were stimulated with VEGF (20 ng/mL) for 10 minutes. F-actin (red) and VE-cadherin (VE-cad.; green) staining is shown. Arrowheads indicate the gaps between adjacent ECs and lack of VE-cadherin. Nuclei were labeled with TOPRO-3 (TOP.; blue). (B) Suppression of VEGF-mediated VE-cadherin internalization by apelin. HUVEC cell surface VE-cadherin labeled by anti–VE-cadherin antibody and stimulated with VEGF (20 ng/mL) in the presence (apelin) or absence (control) of apelin (100 ng/mL). VE-cadherin internalization was visualized in fixed cells using secondary fluorescent antibodies (green). To confirm the internalization of VE-cadherin, cell surface bound VE-cadherin antibodies were removed by a mild acid wash before fixation (right 2 lines of images). Nuclei were labeled with TOPRO-3 (blue). (C) The internalization of endogenous VE-cadherin (in the right 2 lines of panel B) was quantified by the fluorescence intensity of secondary antibodies per cell. *P < .01. (D) Western blot analysis of cell surface and cytoplasmic VE-cadherin protein on HUVECs that had been stimulated with VEGF (20 ng/mL) and/or apelin (100 ng/mL) for 20 minutes. The purity of cytoplasmic protein was confirmed by the lack of expression of the cell surface protein N-cadherin. GAPDH expression was analyzed as a control for an unrelated intracellular protein.
When living HUVECs were labeled with anti–VE-cadherin antibody, internalization of VE-cadherin was induced on stimulation with VEGF, as previously reported.45 Only internalized VE-cadherin was visible after acid wash treatment, which removes membrane-bound antibodies (Figure 6B-C). However, on pretreatment with apelin, VEGF no longer induced internalization of VE-cadherin, and membrane-bound VE-cadherin was lost from EC-EC junctions on acid washing (Figure 6B-C). Western blot analysis of extracts of cells stimulated with apelin and/or VEGF demonstrated unaltered total cell-associated VE-cadherin, but VEGF induced the accumulation of VE-cadherin in the cytoplasmic fraction, depleting the membrane fraction. This internalization of VE-cadherin was suppressed by simultaneous addition of apelin (Figure 6D). p120 catenin has been shown to act as a retention factor for VE-cadherin, preventing endocytosis of the transmembrane protein. Although loss of VE-cadherin after treatment with VEGF, impacting on the EC-EC junction, was accompanied by a loss of p120 catenin, apelin treatment together with VEGF resulted in retention of plasma membrane-associated VE-cadherin and p120 catenin (supplemental Figure 7C). Therefore, we concluded that apelin stabilizes VE-cadherin on the cell surface, resulting in promotion of the formation of nonleaky blood vessels.
Nonetheless, histamine stimulation did not promote the internalization of VE-cadherin in cultured HUVEC monolayers (supplemental Figure 9A-B). This suggests that additional mechanisms contribute to the suppressive effects of apelin on gap formation.
Discussion
Here we report that endogenous apelin is required for the recovery from ischemia and that additional apelin induction together with VEGF significantly restores ischemia damage by generating enlarged blood vessels in the ischemic muscle. Interestingly, VEGF-mediated hyperpermeability is almost completely suppressed by simultaneous induction of apelin. Therefore, we concluded that apelin is one of the factors inducing enlarged and nonleaky blood vessels.
We previously reported that the apelin/APJ system spatially and temporally modulates caliber size enlargement during embryogenesis.24 APJ expression in adult ECs was weak, but VEGF induced its expression on HUVECs.24 Our present results also showed that APJ expression on ECs is induced in the hind limb ischemia model, and this is consistent with the up-regulation of VEGF in ischemic muscle, suggesting the induction of APJ expression on ECs by VEGF. Moreover, in the retina, it has been reported that strong APJ expression was observed temporally in the radial vessels from day 3 to day 12 after birth, but APJ expression on ECs was attenuated later.46 Thus, these data strongly suggest that APJ expression is induced when angiogenesis is taking place under conditions of tissue hypoxia. In the case of apelin, we previously reported that its expression is up-regulated in ECs on stimulation with Ang-124 ; however, recently it has been suggested that apelin expression is induced by tissue hypoxia, and this is consistent with evidence for the existence of hypoxia-inducible factor binding sites in the promoter sequences of the apelin gene.26 A function for apelin/APJ during postnatal angiogenesis in physiologic or pathologic situations, especially associating with tissue hypoxia, is strongly implied by these data.
It is widely thought that maturation of blood vessels is closely related to cell contact between mural cells and ECs and that this process is induced by Ang-1 produced from mural cells recruited around ECs by platelet-derived growth factor, resulting in initiation of cell adhesion between mural cells and ECs.47 So far, however, it has not been established when enlargement of blood vessels is induced for the adjustment of blood flow responding to tissue demands for oxygen and nutrients. Exchange of gas and nutrients is mainly regulated in the capillary bed. Therefore, caliber size regulation associated with gas and nutrient perfusion must be regulated at the capillary level. Generally, on adhesion between mural cells and ECs, proliferation of the latter is suppressed by angiogenic factors, such as transforming growth factor-β,47 and this event finalizes angiogenesis. Because it is thought that caliber size regulation is exerted during angiogenesis, blood vessels formed by EC alone may change their caliber before contact with mural cells and cell adhesion takes place. In K14-apelin Tg mice, caliber size of arteriola and venula in the dermis was not different from WT mice. This may be because those blood vessels are already covered with mural cells during embryogenesis before activation of the K14 promoter. In this case, apelin overexpressed in the skin would not affect the ECs of those vessels in which APJ expression is attenuated. On the other hand, capillaries in the dermis of K14-apelin Tg mice were significantly enlarged compared with those of WT mice. Because mural cell covering is usually sparse in the capillaries compared with arteriola and venula and sprouting angiogenesis is actively occurring at the capillary level after birth, it is possible that apelin affects capillaries but not arteriola and venula. Taken together with the phenotype of K14-apelin Tg mice and the expression of apelin and APJ on ECs under tissue hypoxia, we conclude that the target vessels of apelin are immature blood vessels at the stage before mural cell adhesion to ECs.
We previously suggested that EC-EC aggregation might be an important mechanism for enlargement of blood vessels mediated by apelin during embryogenesis.24 At present, it is not clear how larger blood vessels are induced by apelin during adult angiogenesis, but this might be similar to the mechanism used in the embryo.
In addition to mere enlargement, we found that apelin induces nonleaky blood vessels. A requirement for endogenous apelin for the suppression of vascular permeability caused by factors, such as VEGF, histamine, and inflammatory agents, was noted in apelin-deficient mice; we confirmed this effect in K14-apelin Tg mice. Tissue edema is caused by lymphatic dysfunction and hemodynamic disorder as well as vascular hyperpermeability. We observed that lymphatic vessel formation was not greatly different in WT and apelin−/− mice (data not shown). It has been reported that apelin- or APJ-deficient mice have similar, normal blood pressure.48 Although we cannot completely exclude the possibility of a contribution of apelin function to lymphatic vessel integrity and hemodynamics, its direct effects on vascular permeability were confirmed only using in vitro endothelial monolayer assays (supplemental Figure 6). These results suggest a benefit of apelin in therapeutic angiogenesis when combined with several proangiogenic factors. VEGF has the disadvantage of promoting edema, but apelin may prevent this by suppressing vascular leakiness.13 Indeed, we found that apelin inhibited VEGF-mediated hyperpermeability of newly developed blood vessels in the hind limb ischemia model. Previous reports showed that apelin activates src-dependent protein kinase C activation and blocks increased cyclic adenosine monophosphate accumulation by reducing the intracellular Ca2+ concentration.28,49 Furthermore, apelin also induces the phosphorylation of myosin light chains in vascular smooth muscle cells.50 From those data, one would not expect apelin to suppress vascular leakage, but rather to promote it. However, our results are completely at odds with this predicted function of apelin in vascular permeability. We are unable to explain this apparent inconsistency at present; however, suppression of VE-cadherin internalization by apelin, as shown here, may surpass the signaling of gap formation by stabilizing cell-to-cell junctions. The molecular mechanism of how apelin might stabilize VE-cadherin localization in plasma membranes is also not clear at present. It is well known that VEGF-mediated hyperpermeability and VE-cadherin internalization are suppressed by Ang-1.16,17 Recently, it has been reported that this process involves the activation of RhoA by Ang-1 and its subsequent association with mDia, a RhoA downstream target.45 We found apelin induction in ECs via Tie2 activation by Ang-1. Therefore, similar signaling pathways may be involved in the suppression of VE-cadherin internalization by apelin/APJ; however, the precise molecular mechanism requires elucidation in future work.
Recently, the effectiveness of FGF1 gene therapy for patients with severe limb ischemia was demonstrated in clinical trials; apelin might also be useful in developing gene therapy for such patients in combination with FGF1 or VEGF.7 Especially for diabetic patients, who often have severe limb ischemia, it is anticipated that apelin/FGF or apelin/VEGF gene therapy will be particularly beneficial. Further studies will be required to confirm the effect of apelin together with FGF or VEGF for treating ischemia using diabetic mouse models.
As angiogenesis is controlled by molecular multistep processes,13 the use of a single growth factor may be insufficient to promote a functional vascular system response in therapeutic angiogenesis. Although several factors or cells can initiate the formation of neovessels by promoting proliferation and migration of ECs, stabilization of EC-EC contacts must be considered essential for producing mature vessels. As shown in the present study, our data suggest a new model for generating functional nonleaky blood vessels by regulating caliber size and permeability of newly formed vessels by apelin in ischemic disease.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Y. Oike for supplying K14 promoter DNA, Dr S. Fukuhara for supplying VE-cadherin expression plasmid, and Dr N. Mochizuki, Ms K. Fukuhara, and Ms N. Fujimoto for technical assistance.
This work was supported by grants from the Japan Society for the Promotion of Science and Research, a Fellowship from the Japan Society for the Promotion of Science for Young Scientists, and a grant from the Ministry of Education, Science, Sports, and Culture of Japan.
Authorship
Contribution: H.K. and N.T. designed and performed research, analyzed data, and wrote the paper; and H.N. performed research and analyzed data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Nobuyuki Takakura, Department of Signal Transduction, Research Institute for Microbial Diseases, Osaka University, 3-1 Yamada-oka, Suita, Osaka 565-0871, Japan; e-mail: ntakaku@biken.osaka-u.ac.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal