Abstract
Rituximab plus intravenous bolus chemotherapy is a standard treatment for immunocompetent patients with B-cell non-Hodgkin lymphoma (NHL). Some studies have suggested that rituximab is associated with excessive toxicity in HIVassociated NHL, and that infusional chemotherapy may be more effective. We performed a randomized phase 2 trial of rituximab (375 mg/m2) given either concurrently before each infusional etoposide, vincristine, doxorubicin, cyclophosphamide, and prednisone (EPOCH) chemotherapy cycle or sequentially (weekly for 6 weeks) after completion of all chemotherapy in HIV-associated NHL. EPOCH consisted of a 96-hour intravenous infusion of etoposide, doxorubicin, and vincristine plus oral prednisone followed by intravenous bolus cyclophosphamide given every 21 days for 4 to 6 cycles. In the concurrent arm, 35 of 48 evaluable patients (73%; 95% confidence interval, 58%-85%) had a complete response. In the sequential arm, 29 of 53 evaluable patients (55%; 95% confidence interval, 41%-68%) had a complete response. The primary efficacy endpoint was met for the concurrent arm only. Toxicity was comparable in the 2 arms, although patients with a baseline CD4 count less than 50/μL had a high infectious death rate in the concurrent arm. We conclude that concurrent rituximab plus infusional EPOCH is an effective regimen for HIV-associated lymphoma. This study is registered at http://clinicaltrials.gov as NCT00049036.
Introduction
Rituximab is a chimeric monoclonal antibody that is directed against CD20, a B-cell antigen expressed by normal B lymphocytes and also by approximately 85% of non-Hodgkin lymphoma.1 The combination of rituximab plus chemotherapy is now considered standard therapy for diffuse, large B-cell lymphoma (DLBCL) based on several trials that have consistently demonstrated improved complete response (CR), failure-free survival, and overall survival in older2,3 and younger4 immunocompetent patients with DLBCL who received rituximab plus chemotherapy compared with chemotherapy alone. In several trials, the standard chemotherapy regimen consisted of intravenous bolus cyclophosphamide, doxorubicin, and vincristine plus oral prednisone (CHOP), or CHOP-like regimens. At least one report also suggests benefit when rituximab is combined with more intensive cytotoxic regimens for aggressive B-cell lymphoma.5
For patients with lymphoma associated with HIV infection, however, the benefits of rituximab are less certain. HIV-associated lymphoma tends to present with advanced-stage disease, aggressive histology, and compromised performance status, immune function, and hematopoietic reserve; these factors contribute to a poorer prognosis compared with B-cell lymphoma in immunocompetent persons.6,7 In a previous trial performed by the AIDS Malignancy Consortium, the addition of rituximab to CHOP chemotherapy (R-CHOP) was associated with a significantly higher risk of treatment associated death compared with CHOP alone (14% vs 2%; P = .035), and resulted in comparable CR rates (58% vs 47%; P = .147) and survival.8 This raised concerns about the safety and efficacy of R-CHOP in patients with HIV-associated lymphoma.9
Infusional administration of cytotoxic therapy has also been explored as a potential strategy in patients with poor-risk lymphoma,10-13 including HIV-associated lymphoma when used as first-line therapy. For example, Little et al evaluated a 96-hour continuous intravenous infusional regimen of etoposide, vincristine, doxorubicin, cyclophosphamide, and prednisone (EPOCH) in 39 patients with HIV-associated lymphoma; 29 patients (74%) achieved a CR, and after a median follow-up of 53 months, the median progression-free survival (PFS) rate was 73%, and overall survival rate was 60%.14 Other studies evaluating a 96-hour infusion of cyclophosphamide, doxorubicin, and etoposide in HIV-associated lymphoma produced similar results,15 with outcome improved for patients treated in the highly active antiretroviral therapy (HAART) era.16 In addition, the safety and efficacy of rituximab plus infusional chemotherapy17 or CHOP chemotherapy18,19 in patients with HIV-associated lymphoma has also been demonstrated in other studies, suggesting that HIV-infected patients may derive similar benefit from rituximab.20
Based on these considerations, the AIDS Malignancy Consortium (AMC) initiated a randomized phase 2 trial evaluating the safety and efficacy of infusional EPOCH chemotherapy given either concurrently with rituximab every 3 weeks for up to 6 cycles, or EPOCH given alone every 3 weeks for up to 6 cycles sequentially before initiating weekly rituximab for up to 6 doses. This design would facilitate the evaluation of each experimental treatment arm independently compared with historical data. The trial was designed to distinguish between a CR rate of 50% expected for standard therapy and 75%, which was defined a priori as promising. This design also permitted a direct comparison of the toxicity in each treatment arm.
Methods
Eligibility criteria
Eligibility criteria included previously untreated histologically or cytologically documented aggressive CD20+ B-cell non-Hodgkin lymphoma, including DLBCL, Burkitt/Burkitt-like lymphoma, or other aggressive lymphomas associated with HIV infection. Other criteria included stage II to IV disease (or stage I disease with an elevated serum lactate dehydrogenase), Eastern Cooperative Oncology Group performance status of 0 to 2, age 18 years or older, and adequate organ function similar to a previous AMC lymphoma trial; exclusion criteria were also similar to the previous trial.8 All patients were also required to be able to provide written informed consent.
Rituximab, EPOCH, supportive care, and clinical evaluation
Details regarding treatment program are summarized in Table 1. Response was defined by the International Response Criteria for Non-Hodgkin Lymphoma (which uses anatomic but not functional imaging).21 Toxicity was graded according to the National Cancer Institute Common Toxicity Criteria (Version 2.0). Response was evaluated after every 2 cycles of EPOCH therapy (with computerized tomography of the chest, abdomen, and pelvis) and continued for 2 cycles beyond achieving a CR (for a minimum of 4 and maximum of 6 cycles), including after completion of R-EPOCH in the concurrent arm, and after completion of EPOCH alone and by rituximab alone in the sequential arm. All patients were required to have bone marrow biopsy and lumbar puncture for cerebrospinal fluid cytologic examination at baseline. A repeat bone marrow biopsy was required if the original study demonstrated lymphomatous marrow involvement, and if the physical examination and imaging studies were consistent with a complete response.
Treatment regimen and supportive care
| Drug . | Dose and route . | Duration . | Schedule . | Dose modifications for toxicity . |
|---|---|---|---|---|
| Rituximab | 375 mg/m2 IV infusion | Over 2-6 h | Before each EPOCH cycle (arm A) or weekly times 6 weeks after EPOCH completed (arm B) | Skin/mucositis: discontinue if attributed to rituximab |
| EPOCH | ||||
| Etoposide | 50 mg/m2/day by continuous IV infusion | 4 days (96 h) | Every 3 weeks | Hematolotogic*: reduce by 25%Renal: reduce to 25% if creatinine clearance < 40 mL/min |
| Doxorubicin | 10 mg/m2/day by continuous IV infusion | 4 days (96 h) | Every 3 weeks | Hematologic*: reduce by 25%Hepatic: reduce to 25% if direct bilirubin > 2.5 mg/dL |
| Vincristine | 0.4 mg/m2/day by continuous IV infusion | 4 days (96 h) | Every 3 weeks | Neurotoxicity: obstipation or unable to walk on heels, reduce to 0.3 mg/m2/day; difficulty ambulating, discontinueHepatic: reduce to 25% if direct bilirubin > 2.5 mg/dLRenal: reduce to 25% if creatinine clearance < 40 mL/min |
| Prednisone | 60 mg/m2 per day orally | 5 days | Every 3 weeks | |
| Cyclophosphamide | Cycle 1 187 mg/m2 IV if CD4 < 100/μL375 mg/m2 IV if CD4 > 100/μL | 60-minute infusion | Every 3 weeks after completion of etoposide, doxorubicin, vincristine infusion | Cycle 2-6: escalate dose in increments of 187 mg/m2 per cycle if neutrophil nadir > 500/μL and platelet nadir > 25 000/μL in preceding cycleReduce dose in increments of 187 mg/m2 if the nadir neutrophil count was < 500/μL or platelet nadir < 25 000/μL in preceding cycle |
| Supportive care | ||||
| Filgrastim (or pegfilgrastim) | 5 μg/kg subcutaneous injection (or pegfilgrastim 6 mg) ∼ 24 h after EPOCH completed | Daily until neutrophil recovery (or once for pegfilgrastim) | Begin on day 6 after EPOCH completed | |
| Trimethorprim-sulfamethoxazole | 160-800 mg PO | Continuous | 3× weekly (eg, Monday, Wednesday, Friday) | |
| Fluconazole | 100 mg PO | Continuous | Daily | |
| Ciprofloxacin | 500 mg PO BID (or alternative quinolone and dose at discretion of physician) | At least 8 days each cycle | Begin on day 8 of each cycle, continue until day 15 or postnadir neutrophil count ≥ 1000/μL |
| Drug . | Dose and route . | Duration . | Schedule . | Dose modifications for toxicity . |
|---|---|---|---|---|
| Rituximab | 375 mg/m2 IV infusion | Over 2-6 h | Before each EPOCH cycle (arm A) or weekly times 6 weeks after EPOCH completed (arm B) | Skin/mucositis: discontinue if attributed to rituximab |
| EPOCH | ||||
| Etoposide | 50 mg/m2/day by continuous IV infusion | 4 days (96 h) | Every 3 weeks | Hematolotogic*: reduce by 25%Renal: reduce to 25% if creatinine clearance < 40 mL/min |
| Doxorubicin | 10 mg/m2/day by continuous IV infusion | 4 days (96 h) | Every 3 weeks | Hematologic*: reduce by 25%Hepatic: reduce to 25% if direct bilirubin > 2.5 mg/dL |
| Vincristine | 0.4 mg/m2/day by continuous IV infusion | 4 days (96 h) | Every 3 weeks | Neurotoxicity: obstipation or unable to walk on heels, reduce to 0.3 mg/m2/day; difficulty ambulating, discontinueHepatic: reduce to 25% if direct bilirubin > 2.5 mg/dLRenal: reduce to 25% if creatinine clearance < 40 mL/min |
| Prednisone | 60 mg/m2 per day orally | 5 days | Every 3 weeks | |
| Cyclophosphamide | Cycle 1 187 mg/m2 IV if CD4 < 100/μL375 mg/m2 IV if CD4 > 100/μL | 60-minute infusion | Every 3 weeks after completion of etoposide, doxorubicin, vincristine infusion | Cycle 2-6: escalate dose in increments of 187 mg/m2 per cycle if neutrophil nadir > 500/μL and platelet nadir > 25 000/μL in preceding cycleReduce dose in increments of 187 mg/m2 if the nadir neutrophil count was < 500/μL or platelet nadir < 25 000/μL in preceding cycle |
| Supportive care | ||||
| Filgrastim (or pegfilgrastim) | 5 μg/kg subcutaneous injection (or pegfilgrastim 6 mg) ∼ 24 h after EPOCH completed | Daily until neutrophil recovery (or once for pegfilgrastim) | Begin on day 6 after EPOCH completed | |
| Trimethorprim-sulfamethoxazole | 160-800 mg PO | Continuous | 3× weekly (eg, Monday, Wednesday, Friday) | |
| Fluconazole | 100 mg PO | Continuous | Daily | |
| Ciprofloxacin | 500 mg PO BID (or alternative quinolone and dose at discretion of physician) | At least 8 days each cycle | Begin on day 8 of each cycle, continue until day 15 or postnadir neutrophil count ≥ 1000/μL |
IV indicates intravenous; PO, by mouth; and BID, twice a day.
Toxicity calling for dose reduction of doxorubicin and etoposide included a neutrophil nadir less than 500/μL for at least 3 days or platelets less than 25 000/μL for at least 3 days if this occurs during a cycle in which no cyclophosphamide was given; otherwise, doxorubicin/etoposide was not reduced.
A 24-hour supply of vincristine, doxorubicin, and etoposide was administered in 500 mL of 0.9% sodium chloride and delivered by a portable infusion pump given by a central venous catheter. Rituximab (375 mg/m2) was given either before each EPOCH course in the concurrent arm (arm A), or sequentially after the completion of all EPOCH on a weekly basis for 6 consecutive weeks (arm B) beginning approximately 3 weeks after the last EPOCH cycle when there was satisfactory recovery from toxicity. Rituximab (Rituxan, Genentech) was provided by the National Cancer Institute for all patients. EPOCH treatment cycles were repeated every 3 weeks if the neutrophil count was at least 1000/μL, platelets at least 50 000/μL, and the patient had recovered from nonhematologic toxicity. EPOCH dose modification was performed as previously described and as summarized in Table 1.14 Central nervous system prophylaxis was given at the discretion of the treating physician and was recommended for persons with small noncleaved cell histology, bone marrow involvement, paranasal sinus involvement, testicular involvement, or epidural disease. The recommended central nervous system prophylaxis regimen included intrathecal injection of either cytarabine (50 mg) or methotrexate (12 mg) weekly for 4 consecutive weeks during cycle 1 of EPOCH. Patients with positive baseline cerebrospinal fluid cytology were permitted to remain on study and receive an intrathecal chemotherapy regimen of the physician's choice.
Supportive care used is summarized in Table 1 and included antibacterial prophylaxis for those in the concurrent arm (using a fluoroquinolone of the investigator's choice on days 8-15 of each cycle or longer). Patients in the sequential arm were also required to use quinolone prophylaxis if there were high-risk features for infection, including a baseline CD4 count of less than 100/μL or decreasing below that level during therapy, a nadir neutrophil count of less than 500/μL in a previous or current cycle, or a nadir neutrophil count of less than 1000/μL associated with fever of at least 38.0°C occurring in a previous cycle. A complete blood count was obtained before beginning each cycle, and on or about days 8 and 15 of EPOCH therapy to document adequate recovery of the neutrophil count to at least 1000/μL before the quinolone was discontinued.
The use of antiretroviral therapy was left to the choice of the treating physician. For patients who were never previously treated with antiretroviral agents or who were not receiving them within 4 weeks of registration, it was recommended that concurrent antiretroviral therapy be withheld until completion of EPOCH chemotherapy. For those who were taking antiretrovirals at the time of registration and were on a stable, well-tolerated regimen that was producing adequate viral suppression, it was recommended that patients remain on antiretroviral therapy.
Randomization, statistical considerations and analysis, and protocol amendment
Randomization was performed centrally by the AMC Statistical Office. Stratification factors included CD4 count (< 100/μL vs ≥ 100/μL), age-adjusted International Prognostic Index (0 or 1 vs 2 or 3 risk factors), and planned concurrent antiretroviral therapy (yes vs no). The primary endpoint was CR rate, including unconfirmed and confirmed CR as defined by the International Response Criteria (which uses anatomic but not functional imaging).21 The primary objective was to distinguish between a CR rate of 75% with experimental therapy compared with 50% typically expected with standard therapy for HIV-associated lymphoma. Because neither of the regimens evaluated in this trial was considered standard therapy, both treatment arms were regarded as experimental. For each treatment arm, a sample size of 31 eligible and evaluable patients was planned based on testing the null hypothesis that the CR rate was 50% against the alternative that it is 75% at the 2-sided 0.10 significance level with power of 0.90. Assuming a 10% ineligible or unevaluable for response, it was projected that 35 patients in each arm were required. All analyses were performed using Statistical Analysis System software. An early stopping rule was also used that was designed to interrupt accrual if the incidence of treatment-associated deaths exceeded 10% for either arm.
After accrual of 35 patients, an error in the protocol document was found that resulted in omission of the cyclophosphamide dose escalation. The study was suspended to accrual in March 2004, the protocol corrected, and all patients were informed. The study reopened in May 2004 after the amendment to assure that approximately 70 total patients (35 per arm) would be enrolled after protocol correction to include cyclophosphamide dose escalation as originally described for the EPOCH regimen by Little et al.14 The study completed its accrual goal in April 2006. The amended analysis plan indicated that the primary efficacy analysis for CR rate would be based on patients treated after the amendment and that efficacy and toxicity would also be analyzed for the entire study population. PFS, time to progression (TTP), and overall survival were estimated using the method of Kaplan and Meier. PFS was defined as the time between registration and either relapse or progression of lymphoma or death from any cause. TTP was defined as time to progression or relapse of lymphoma, with deaths from other causes censored.
Informed consent and regulatory approval
The study was reviewed and approved by the Cancer Evaluation Therapy Program of the National Cancer Institute, and by the institutional review board at each participating institution. All patients provided written informed consent in accordance with the Declaration of Helsinki.
Results
Patient characteristics
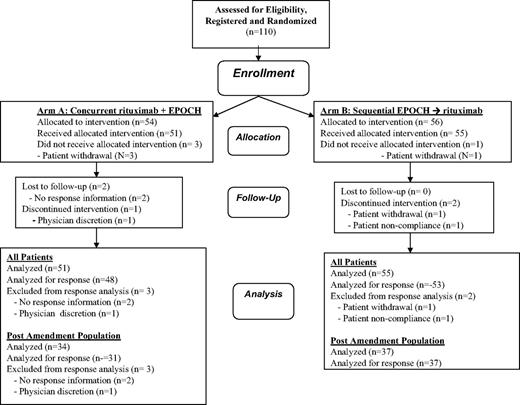
A total of 110 patients were enrolled at 20 sites between December 2002 and April 2006, of whom 106 were treated and included in this analysis (Figure 1); 35 treated patients were enrolled before the amendment and 71 were enrolled after the amendment. The characteristics of the 106 treated patients are shown in Table 2, including the characteristics of the preamendment, postamendment, and total population in each treatment arm. The populations were generally comparable with respect to gender, median age, concurrent antiretroviral therapy, histology (as determined by the local institutional pathologist), and number of age-adjusted International Prognostic Index risk factors. The populations were also comparable with regard to race and ethnicity. With regard to ethnicity, 30 patients (28.3%) were Hispanic (and the remaining were non-Hispanic); with regard to race, 69 were white (65.1%), 28 were black (26.4%), 4 were native Pacific Islanders (3.8%), 3 were Asian (2.8%), and 2 were characterized as other (1.9%). However, significantly more patients in the postamendment cohorts, including both arms, received concurrent antiretroviral therapy (77% vs 57%, P = .041). In addition, patients in the concurrent arm had a numerically (but not significantly) lower median CD4 count in the postamendment compared with the preamendment cohort (median, 153/μL vs 295/μL), a trend that was not evident in the sequential arm (median, 194/μL vs 212/μL). However, for the total population, median CD4 count was generally similar in the concurrent and sequential arms (median 181/μL vs 194/μL).
Clinical characteristics of the entire population by treatment arm
| . | Arm A: concurrent R-EPOCH . | Arm B: sequential EPOCH → R . | ||||
|---|---|---|---|---|---|---|
| Preamendment . | Postamendment . | All patients . | Preamendment . | Postaamendment . | All patients . | |
| No. treated | 17 | 34 | 51 | 18 | 37 | 55 |
| Male sex | 14(82%) | 29(85%) | 43(84%) | 16(89%) | 32(86%) | 48(87%) |
| Median age, y | 45 | 44 | 44 | 44 | 43 | 43 |
| CD4 count | ||||||
| Median, /μL | 295 | 153 | 181 | 212 | 194 | 194 |
| Less than 100/μL | 4(24%) | 12(35%) | 16(31%) | 5(28%) | 12(32%) | 17(31%) |
| Concurrent ARV | 10(59%) | 26(76%) | 36(71%) | 10(56%) | 29(78%) | 39(71%) |
| Local histology | ||||||
| Diffuse large cell | 12(71%) | 23(68%) | 35(69%) | 14(78%) | 30(81%) | 44(80%) |
| Burkitt/Burkitt-like or other* | 5(29%) | 11(32%) | 16(31%) | 4(22%) | 7(19%) | 11(20%) |
| Stage III-IV | 14(82%) | 29(85%) | 43(84%) | 13(72%) | 28(76%) | 41(75%) |
| Elevated LDH | 11(65%) | 25(73%) | 36(71%) | 11(61%) | 25(68%) | 36(65%) |
| ECOG PS 2 | 6(35%) | 8(24%) | 14(27%) | 2(11%) | 9(24%) | 11(20%) |
| Age-adjusted IPI | ||||||
| 0 or 1 risk factors | 6(35%) | 10(29%) | 16(31%) | 6(33%) | 14(38%) | 20(36%) |
| 2 or 3 risk factors | 11(65%) | 24(71%) | 35(69%) | 12(67%) | 23(62%) | 35(64%) |
| . | Arm A: concurrent R-EPOCH . | Arm B: sequential EPOCH → R . | ||||
|---|---|---|---|---|---|---|
| Preamendment . | Postamendment . | All patients . | Preamendment . | Postaamendment . | All patients . | |
| No. treated | 17 | 34 | 51 | 18 | 37 | 55 |
| Male sex | 14(82%) | 29(85%) | 43(84%) | 16(89%) | 32(86%) | 48(87%) |
| Median age, y | 45 | 44 | 44 | 44 | 43 | 43 |
| CD4 count | ||||||
| Median, /μL | 295 | 153 | 181 | 212 | 194 | 194 |
| Less than 100/μL | 4(24%) | 12(35%) | 16(31%) | 5(28%) | 12(32%) | 17(31%) |
| Concurrent ARV | 10(59%) | 26(76%) | 36(71%) | 10(56%) | 29(78%) | 39(71%) |
| Local histology | ||||||
| Diffuse large cell | 12(71%) | 23(68%) | 35(69%) | 14(78%) | 30(81%) | 44(80%) |
| Burkitt/Burkitt-like or other* | 5(29%) | 11(32%) | 16(31%) | 4(22%) | 7(19%) | 11(20%) |
| Stage III-IV | 14(82%) | 29(85%) | 43(84%) | 13(72%) | 28(76%) | 41(75%) |
| Elevated LDH | 11(65%) | 25(73%) | 36(71%) | 11(61%) | 25(68%) | 36(65%) |
| ECOG PS 2 | 6(35%) | 8(24%) | 14(27%) | 2(11%) | 9(24%) | 11(20%) |
| Age-adjusted IPI | ||||||
| 0 or 1 risk factors | 6(35%) | 10(29%) | 16(31%) | 6(33%) | 14(38%) | 20(36%) |
| 2 or 3 risk factors | 11(65%) | 24(71%) | 35(69%) | 12(67%) | 23(62%) | 35(64%) |
ARV indicates antiretroviral therapy; ECOG PS, Eastern Cooperative Oncology Group performance status; and IPI, International Prognostic Index (adverse risk factors included stage III or IV disease, elevated serum lactate dehydrogenase above normal, and Easter Cooperative Oncology performance status 2).
For the Burkitt/Burkitt-like/other category, this included 15 of 16 patents with Burkitt or Burkitt-like lymphoma in arm A and 8 of 11 in arm B; other cases included malignant lymphoma, not otherwise specified.
Treatment administered
A total of 587 EPOCH cycles were given to all 106 patients, including 233 in the concurrent arm (median, 5; range, 1-6 cycles per patient) and 254 in the sequential arm (median, 6; range, 1-6 per patient). Some dose reduction of EPOCH was required for 15 patients (29%) treated in arm A and 17 patients (31%) treated in arm B. The median relative dose intensity was comparable in arms A and B, averaging, respectively 100% and 99% for cyclophosphamide (relative to the planned cyclophosphamide dose in cycle 1), 100% and 99% for etoposide, 98% and 98% for doxorubicin, and 97% and 92% for vincristine. Cyclophosphamide was not given in the second or subsequent cycles (because of protocol specified dose reduction stipulated in Table 1) in 15 patients (29 treatment cycles) in the concurrent arm and 12 patients (26 treatment cycles) in the sequential arm. All patients in the concurrent arm received rituximab (median, 5 doses; range, 1-7 doses), whereas only 28 patients in the concurrent arm (51%) received rituximab (median, 6 doses; range, 2-6 doses for those who received it). Of the 27 patients in the sequential arm who did not receive rituximab, reasons included disease progression (N = 8), death (N = 4), patient withdrawal or noncompliance (N = 4), physician discretion (N = 4), adverse event (N = 1), or other unspecified reasons (N = 6, of whom 2 had a partial response [PR] and 4 had a CR).
For those treated after the amendment, a total of 329 cycles were given to 71 patients, including 156 in the concurrent arm (median, 5; range, 1-6 cycles per patient) and 173 in the sequential arm (median, 6; range, 1-6 per patient). Some dose reduction of EPOCH was required for 10 patients (29%) treated in arm A and 15 patients (41%) treated in arm B. For the postamendment population, the median relative dose intensity was also comparable in arms A and B, averaging, respectively, 118% and 102% for cyclophosphamide (relative to the planned cyclophosphamide dose in cycle 1), 100% and 98% for etoposide, 100% and 96% for doxorubicin, and 97% and 88% for vincristine. For all patients treated after the amendment, the protocol stipulated escalation in the cyclophosphamide dose if the neutrophil nadir were more than 500/μL and platelet nadir were more than 25 000/μL. Patients treated before the amendment were generally not dose escalated, although some of the patients were escalated after sites were notified. After the protocol amendment, 16 of 34 patients (47%) in the concurrent arm and 18 of 37 patients (49%) in the sequential arm had cyclophosphamide dose escalation.
Response data
Response data are shown in Table 3 for the preamendment, postamendment, and total populations. Five patients were considered unevaluable for response in the entire study population (Figure 1) because either response data were unknown (n = 2 in arm A), physician withdrawal from therapy (n = 1 in arm A), patient withdrawal (n = 1 in arm B), or patient noncompliance (n = 1 in arm B). In the concurrent arm, 48 of 51 treated patients were evaluable for response (3 were unevaluable for response after amendment). In the sequential arm, 53 of 55 treated patients were evaluable for response (2 were unevaluable for response before amendment).
Response data by treatment arm
| . | Arm A, concurrent R-EPOCH . | Arm B, sequential EPOCH → R . |
|---|---|---|
| Preamendment | ||
| No. treated/evaluable | 17/17 | 18/16 |
| CR or CRu | 14 (82%) | 11 (69%) |
| 95% CI | 57%-96% | 41%-89% |
| CR + PR | 16 (94%) | 13 (82%) |
| Postamendment | ||
| No. treated/evaluable | 34/31 | 37/37 |
| CR or CRu | 21 (68%) | 18 (49%) |
| 95% CI | 49%-83% | 32%-66% |
| CR + PR | 26 (84%) | 28 (76%) |
| All patients* | 51/48 | 55/53 |
| No. treated/evaluable | ||
| CR or CRu | 35 (73%) | 29 (55%) |
| 95% CI | 58%-85% | 41%-68% |
| CR + PR | 42 (88%) | 41 (77%) |
| . | Arm A, concurrent R-EPOCH . | Arm B, sequential EPOCH → R . |
|---|---|---|
| Preamendment | ||
| No. treated/evaluable | 17/17 | 18/16 |
| CR or CRu | 14 (82%) | 11 (69%) |
| 95% CI | 57%-96% | 41%-89% |
| CR + PR | 16 (94%) | 13 (82%) |
| Postamendment | ||
| No. treated/evaluable | 34/31 | 37/37 |
| CR or CRu | 21 (68%) | 18 (49%) |
| 95% CI | 49%-83% | 32%-66% |
| CR + PR | 26 (84%) | 28 (76%) |
| All patients* | 51/48 | 55/53 |
| No. treated/evaluable | ||
| CR or CRu | 35 (73%) | 29 (55%) |
| 95% CI | 58%-85% | 41%-68% |
| CR + PR | 42 (88%) | 41 (77%) |
CRu indicates complete response (unconfirmed).
In an intention-to-treat analysis in all treated patients, the CR rates 69% in the concurrent arm (95% CI, 54%-81%) and 53% in the sequential arm (95% CI, 39%-66%).
The primary efficacy analysis was based on CR rate in the postamendment population. CR occurred in 21 of 31 evaluable patients (68%; 95% confidence interval [CI], 49%-83%) in the concurrent arm and in 18 of 37 patients (49%; 95% CI, 32%-66%) in the sequential arm. Therefore, the null hypothesis that the CR rate is 50% was rejected in favor of the alternative that is 75% for the concurrent arm, but not the sequential arm; in other words, the concurrent arm met the prespecified efficacy endpoint, whereas the sequential arm did not. The CR rates tended to be lower in the postamendment compared with the preamendment cohorts in both the concurrent arm (68% vs 82%) and sequential arm (49% vs 69%), which may reflect inclusion of patients with more advanced immunosuppression in the postamendment cohort as previously described in “Patient characteristics.”
For the secondary efficacy analysis in the entire population, CR occurred in 35 of 48 evaluable patients (73%; 95% CI, 58%-85%) in the concurrent arm; an additional 7 patients had a PR, yielding an overall response rate of 88%. In the sequential arm, 29 of 53 evaluable patients (55%; 95% CI, 41%-68%) had a CR; an additional 12 patients had a PR, yielding an overall response rate of 77%. For the entire population, the null hypothesis that the CR rate is 50% was rejected in favor of the alternative that is 75% for the concurrent arm (post hoc P = .005, power 0.89) but not the sequential arm (post hoc P = .394). Therefore, the results were similar for the secondary efficacy analysis in the entire population as for the primary efficacy analysis in the postamendment cohort. The results were also similar if an intention to treated analysis, including all treated patients was performed for the entire population, with CR rates being 69% in the concurrent arm (95% CI, 54%-81%) and 53% in the sequential arm (95% CI, 39%-66%). Recurrences occurred in 11 of 64 patients (17%) with CR and 10 of 19 patients (53%) with PR in both arms.
Response by histology and results of central pathology review
When the response rate was analyzed in all treated patients who had DLBCL, CR occurred in 25 of 35 patients (71%; 95% CI, 54%-85%) in the concurrent arm and 20 of 44 patients in the sequential arm (46%; 95% CI, 30%-61%). For those who had Burkitt-like lymphoma and other aggressive subtypes, CR occurred in 10 of 16 patients in the concurrent arm (63%; 95% CI, 35%-85%) and 9 of 11 patients in the sequential arm (82%; 95% CI, 48%-98%).
Central review of pathologic specimens was performed by 2 hematopathologists (A.C. and E.C.) for 58 cases. There was complete concordance between local and central diagnosis in 65%. Only one of 12 tumors (8%) classified as Burkitt/Burkitt-like by the local pathologist was classified as DLBCL centrally, and only 2 of 43 cases (5%) classified as DLCBL locally were classified as Burkitt/Burkitt-like centrally. The majority of cases without exact concordance were not precisely classified centrally because of insufficient material for review.
Adverse events
The incidence of grade 3 or 4 adverse events occurring in at least 5% of patients is shown in Table 4 for the entire population. The overall toxicity profile was comparable in the 2 treatment arms. The most common grade 3 or 4 events in both treatment arms included neutropenia in 42%, infection in 28%, anemia in 16%, thrombocytopenia in 12%, febrile neutropenia in 12%, mucositis in 5%, and neuropathy in 2%. The toxicity profile was similar in the postamendment population (data not shown). Grade 3 or 4 adverse events leading to premature discontinuation of therapy occurred in 3 patients (6%) in arm A (including neutropenia, fever, and hypotension in one patient each) and 3 patients (5%) in arm B (including infection in one patient and hepatitis in 2 patients).
Grade 3 or 4 adverse events occurring in at least 5% of patients for the entire population by treatment arm
| Grade 3 or 4 adverse event . | Arm A, concurrent R-EPOCH (n = 51), no. (%) . | Arm B, sequential EPOCH → R (n = 55), no. (%) . |
|---|---|---|
| Neutropenia | 22 (43) | 22 (40) |
| Anemia | 10 (22) | 7 (13) |
| Thrombocytopenia | 4 (8) | 9 (16) |
| Febrile neutropenia | 8 (16) | 8 (15) |
| Infection | 14 (27) | 16 (29) |
| Neuropathy | 0 | 3 (5) |
| Mucositis | 1 (2) | 4 (7) |
| Grade 3 or 4 adverse event . | Arm A, concurrent R-EPOCH (n = 51), no. (%) . | Arm B, sequential EPOCH → R (n = 55), no. (%) . |
|---|---|---|
| Neutropenia | 22 (43) | 22 (40) |
| Anemia | 10 (22) | 7 (13) |
| Thrombocytopenia | 4 (8) | 9 (16) |
| Febrile neutropenia | 8 (16) | 8 (15) |
| Infection | 14 (27) | 16 (29) |
| Neuropathy | 0 | 3 (5) |
| Mucositis | 1 (2) | 4 (7) |
Treatment-associated deaths were defined as deaths occurring during or within 30 days of treatment from causes other than lymphoma that were judged to be possibly or probably associated with therapy. Treatment-associated deaths did not occur at a rate that triggered study interruption. There were 5 treatment-associated deaths (9.8%) in the concurrent arm and 4 (7.3%) in the sequential arm. Causes of death (and baseline CD4 count) in the concurrent arm included cryptosporidiosis (32/μL), wasting (2/μL), sepsis (75/μL), multisystem failure (42/μL), and JC virus (153/μL); the median time between treatment initiation and death was 2 months (range, 1-5 months). Causes of death in the sequential arm included sepsis in 3 patients (94/μL, 194/μL, 194/μL) and HIV complications (662/μL) in one patient; the median time between treatment initiation and death was 1 month (range, 1-4 months). Although the treatment-associated death rate was comparable in the 2 treatment arms, they were numerically (although not significantly) more common in severely immunosuppressed patients in the concurrent arm, occurring in 3 of 8 patients (38%) with a baseline CD4 count of less than 50/μL in the concurrent arm compared with 0 of 7 in the sequential arm (Fisher exact 2-sided P = .2)
PFS, overall survival, and TTP
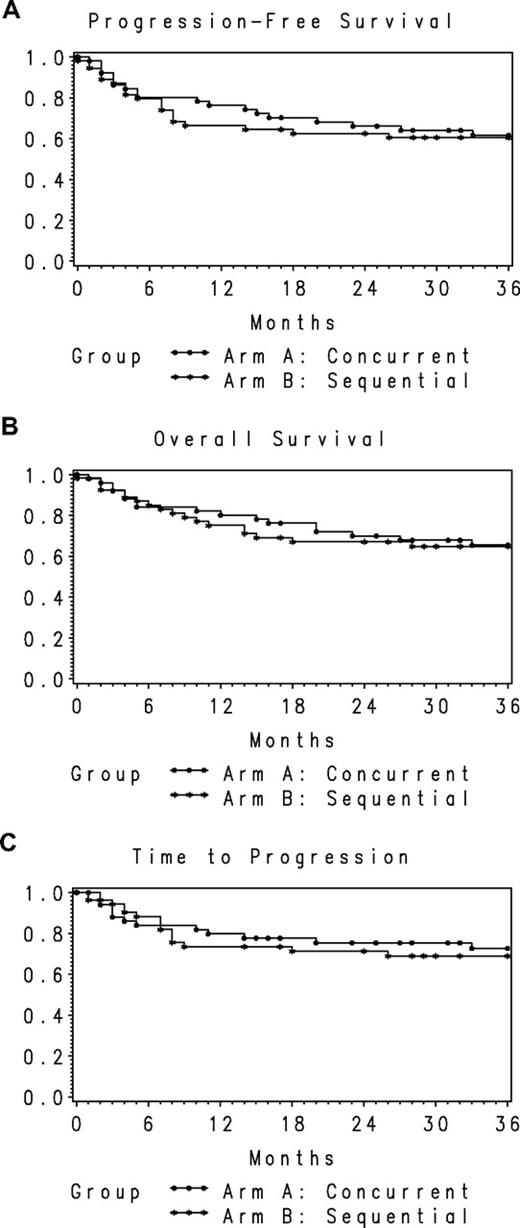
After a median follow-up of 30.0 months (range, 0-68 months) in all treated patients, 17 patients (33%) died in the concurrent arm and 19 (35%) died in the sequential arm. Lymphoma was the cause of death in 9 patients (18%) in the concurrent arm and 10 (16%) in the sequential arm. The 1-year and 2-year PFS rates in the concurrent arm were 78% (95% CI, 67%-90%) and 66% (95% CI, 53%-79%), respectively, and in the sequential arm were 66% (95% CI, 54%-79%) and 63% (95% CI, 50%-76%), respectively (Figure 2A). Two-year overall survival rates were 70% (95% CI, 57%-83%) in the concurrent and 67% (95% CI, 54%-80%) in the sequential arms, respectively (Figure 2B). The 2-year TTP rates in the concurrent and sequential arms were 75% (95% CI, 63.2%-87.5%) and 71% (95% CI, 58.5%-84.0%), respectively, indicating that approximately 70% to 75% of surviving patients were without evidence of progressive lymphoma at 2 years (Figure 2C). Results were similar in the postamendment population. The 2-year PFS rates in the concurrent and sequential arms were 64% (95% CI, 48%-80%) and 60% (95% CI, 44%-75%), respectively. Two-year overall survival rates were 63% (95% CI, 47%-80%) and 66% (95% CI, 51%-82%), respectively.
PFS, overall survival, and TTP for the study population. (A) PFS for the entire population by treatment arm (n = 106). (B) Overall survival for the entire population by treatment arm (n = 106). (C) TTP for the entire population by treatment arm (n = 106).
PFS, overall survival, and TTP for the study population. (A) PFS for the entire population by treatment arm (n = 106). (B) Overall survival for the entire population by treatment arm (n = 106). (C) TTP for the entire population by treatment arm (n = 106).
Discussion
The incidence of B-cell non-Hodgkin lymphoma is increased in HIV-infected persons.22 Since the routine use of HAART in clinical practice beginning in 1996, there have been significant reductions in HIV-associated morbidity and mortality.23,24 Although the incidence of HIV-associated lymphoma has also declined in the HAART era25 and its prognosis has improved compared with the dismal prognosis in pre-HAART era, it remains a common complication of HIV infection that is cured in only 30% to 50% with standard antilymphoma therapy.26
Our trial was designed to determine the efficacy of infusional EPOCH chemotherapy used alone (followed sequentially by rituximab) and infusional EPOCH given concurrently with rituximab in patients with HIV-associated B-cell non-Hodgkin lymphoma. CR rate was chosen as the primary efficacy endpoint because it is a well-accepted short-term surrogate known to be associated with long-term cure, and standard response criteria and response definitions were used. We used what some have referred to as a “selection” or “pick the winner” randomized phase 2 trial design, wherein each arm is regarded as experimental and the efficacy of each arm is evaluated independently and compared with historical data with standard therapy.27 We sought to determine whether either experimental arm was associated with a CR rate of at least 75%, substantially higher than the approximately 50% CR rate typically observed for standard regimens, including CHOP or R-CHOP therapy in a previous AMC trial (AMC 010) performed in the HAART era.8 Although this design does not allow a direct efficacy comparison between the 2 arms, it does facilitate a direct comparison of toxicity in the 2 arms.
First, we found that concurrent rituximab plus infusional EPOCH chemotherapy resulted in a sufficiently high CR rate required to reject that null hypothesis of no difference compared with standard therapy; in other words, the CR rate was significantly higher than would be expected with standard therapy for this arm. Second, the CR rate observed with EPOCH alone (followed sequentially by rituximab) was consistent with CR rates typically observed with standard regimens. Third, although PFS and overall survival rates were similar for the 2 arms, the trial was not adequately powered to detect differences for these endpoints. In addition, some evidence suggests benefit for rituximab when given sequentially after chemotherapy, thereby confounding any evaluation of PFS or overall survival.3 However, the 1-year PFS rates observed for both the concurrent arm (78%; 95% CI, 67%-90%) and sequential arm (66%; 95% CI, 54%-79%) in this trial compare favorably with those observed with either CHOP alone (50%; 95% CI, 39%-61%) or R-CHOP (48%; 95% CI, 32%-64%) in the AMC 010 trial,8 suggesting that infusional EPOCH may have contributed to this improvement compared with historical data. Fourth, the toxicity profile of infusional EPOCH was comparable whether given alone or concurrently with rituximab. Although the treatment-associated death rate in the concurrent arm was 10%, it was similar to the 7% rate in the sequential arm. Moreover, the infectious death rate for concurrent R-EPOCH may be minimized by administering it only in patients with a CD4 count of at least 50/μL.
The results of this trial seem to contradict the results of a prior study conducted by the AMC (AMC010), which found a significantly higher death rate in the concurrent R-CHOP arm compared with CHOP alone. There are several potential explanations for this difference. First, in the prior trial, patients received concurrent rituximab plus CHOP followed by monthly rituximab infusions for 3 months, which may have contributed to late infectious deaths that occurred in approximately 50% of all patients with treatment-associated deaths. Second, patients in the current trial received quinolone prophylaxis, which was not used in the previous trial. Third, patients in the current trial treated with concurrent R-EPOCH had a substantially higher median CD4 count than the R-CHOP arm of the prior AMC trial (median 194/μL vs 128/μL). However, despite the overall acceptable safety profile associated with concurrent R-EPOCH, patients with a very low CD4 count may be better served by treating with chemotherapy alone, followed by rituximab if there is evidence of good response.
A potential concern about this trial was the failure to consistently dose escalate cyclophosphamide as described in the original reports of EPOCH in HIV-associated lymphoma14 and immunocompetent patients with lymphoma,13,28 a factor that may have contributed to a lower CR rate than expected in the sequential arm of our trial. Although this dose escalation may very well be an important factor that contributes to the efficacy of EPOCH in an immunocompetent population or in Burkitt or Burkitt-like lymphoma, we think that the absence of consistent dose escalation did not compromise the efficacy of the sequential arm of our trial for several reasons. First, the trial was amended to assure that a sufficient number of patients received cyclophosphamide dose escalation in the postamendment population. Second, the CR rates were, if anything, lower (although not significantly so) in the postamendment population, including the concurrent arm (67% postamendment vs 82% preamendment) and sequential arm (49% postamendment vs 69% preamendment). Third, other studies involving intensification of cyclophosphamide and other agents have not proven to be superior to CHOP in HIV-associated lymphoma.29 Therefore, we are confident that a therapeutic strategy of EPOCH alone, including cyclophosphamide dose escalation as originally described, is probably not associated with the 75% CR rate we were targeting. In addition, the subtle differences among the preamendment and postamendment populations support our approach of also evaluating efficacy in the total population, and not just in the postamendment population alone, because of the known adverse impact of severe immunosuppression on response and prognosis in HIV-associated lymphoma.30 This also points out the importance of including a concurrent control arm in future trials evaluating novel treatment approaches in HIV-associated lymphoma when a standard potentially curative treatment option is available.
This trial included patients with both DLBCL and Burkitt/Burkitt-like lymphoma, with DLBCL accounting for approximately 74% of the patients treated. Although R-CHOP or R-CHOP–like regimens are commonly used for DLBCL, more intensive regimens are typically recommended for Burkitt/Burkitt-like lymphomas.31,32 For DLBCL, the CR rate appeared to be particularly encouraging for concurrent R-EPOCH arm (71%; 95% CI, 54%-85%) but not for the sequential arm (46%; 95% CI, 30%-61%). For Burkitt/Burkitt-like/other lymphomas, CR rates were encouraging for both the concurrent arm (63%; 95% CI, 35%-85%) and sequential arm (82%; 95% CI, 48%-98%), although the number of patients evaluated was relatively small. Our findings suggest that concurrent R-EPOCH may be a particularly effective regimen for HIV-associated DLBCL and that an EPOCH-containing regimen may be considered a reasonable alternative for patients with HIV-associated Burkitt/Burkitt-like lymphoma who may not be suitable candidates for more intensive regimens. Given the importance of cyclophosphamide dose in Burkitt/Burkitt-like lymphoma,33 additional studies in Burkitt/Burkitt-like lymphoma of rituximab plus EPOCH, including cyclophosphamide dose escalation, are warranted.
It has recently been shown that, among immunocompetent patients, DLBCL is a heterogeneous disease that may be further characterized into germinal center (GC) and non-GC subtypes that have differing prognoses that arrive by distinct genetic pathways.34-36 In addition, certain markers have been associated with prognosis, including proliferation index (measured by expression of Ki-67) and others.37 We have recently reported that, in a subgroup of 82 patients with DLBCL derived from this trial and in a previous trial including CHOP (used alone or in combination with rituximab), there was no significant difference in survival, CD4 count, or Epstein-Barr virus expression among patients with the GC and non-GC subtypes.38 The only predictive immunohistochemical marker was found to be Ki-67, wherein a higher proliferation index was associated with improved survival. These results suggest that patients with DLBCL and high proliferation index, a characteristic that is usually associated with Burkitt/Burkitt-like lymphoma, may be adequately treated with R-EPOCH therapy.
Our findings are also consistent with the results of other trials evaluating rituximab plus infusional chemotherapy in aggressive grade lymphoma, including EPOCH in immunocompetent patients,27 or infusional cyclophosphamide, doxorubicin, and etoposide in HIV-positive patients.17 The latter study included 74 patients with HIV-associated B-cell lymphoma; the CR rate was 70%, lethal treatment-associated toxicity rate was 6%, and the estimated 2-year PFS was 59%, similar to our study. Given that rituximab is known to sensitize lymphoma cells to cytotoxic agents in vitro39 and has a long-half life of approximately 7 days,1 infusional administration of cytotoxic therapy may offer greater potential for therapeutic synergy than the same agents given by a conventional intravenous bolus route. The failure to administer rituximab in approximately 50% of those in the sequential arm provides further evidence supporting concurrent delivery with EPOCH. These findings support the design of a phase 3 trial comparing concurrent R-EPOCH with R-CHOP in immunocompetent patients with DLBCL (#NCT00118209), which provides level 1 evidence supporting infusional EPOCH should the trial demonstrate improved efficacy for this arm. In the interim, this report provides level 2 evidence supporting the use of concurrent R-EPOCH in patients with HIV-associated lymphoma, especially DLBCL.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Department of Health and Human Services (grant U01 CA121947; R.M.).
The authors fondly acknowledge and remember Dr William Harrington, who made an important contribution to this work and who died unexpectedly on January 29, 2009. Dr Harrington was an outstanding scientist, clinician, scholar, and friend who will be missed by his colleagues in the AIDS Malignancy Consortium.
National Institutes of Health
Authorship
Contribution: J.A.S. wrote the manuscript; J.A.S., J.Y.L., and L.D.K. wrote the clinical protocol; J.Y.L. performed data and statistical analysis; R.M. provided administrative support and oversight; E.C. and A.C. performed pathologic review of tumor specimens; J.A.S., L.D.K., A.M.L., J.C.R., R.F.A., W.W., D.A., A.N., D.H.H., J.V.R., B.J.D., S.C.R., M.H.S., L.L., L.R., E.C., and A.C. contributed subjects to the trial; and all authors approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A complete list of members of the AIDS Malignancy Consortium can be found in the supplemental Appendix, available on the Blood website; see the Supplemental Materials link at the top of the online article.
Correspondence: Joseph A. Sparano, Montefiore Medical Center-Weiler Division, Department of Oncology, 2 South, Rm 47-48, 1825 Eastchester Rd, Bronx, NY 10461; e-mail: jsparano@montefiore.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal