Heparanase enhances shedding of syndecan-1 (CD138), and high levels of heparanase and shed syndecan-1 in the tumor microenvironment are associated with elevated angiogenesis and poor prognosis in myeloma and other cancers. To explore how the heparanase/syndecan-1 axis regulates angiogenesis, we used myeloma cells expressing either high or low levels of heparanase and examined their impact on endothelial cell invasion and angiogenesis. Medium conditioned by heparanase-high cells significantly stimulated endothelial invasion in vitro compared with medium from heparanase-low cells. The stimulatory activity was traced to elevated levels of vascular endothelial growth factor (VEGF) and syndecan-1 in the medium. We discovered that the heparan sulfate chains of syndecan-1 captured VEGF and also attached the syndecan-1/VEGF complex to the extracellular matrix where it then stimulated endothelial invasion. In addition to its heparan sulfate chains, the core protein of syndecan-1 was also required because endothelial invasion was blocked by addition of synstatin, a peptide mimic of the integrin activating region present on the syndecan-1 core protein. These results reveal a novel mechanistic pathway driven by heparanase expression in myeloma cells whereby elevated levels of VEGF and shed syndecan-1 form matrix-anchored complexes that together activate integrin and VEGF receptors on adjacent endothelial cells thereby stimulating tumor angiogenesis.
Introduction
Enzymatic remodeling of heparan sulfate proteoglycans has emerged as a key mechanism for controlling tumor cell behavior.1 For example, cell membrane bound heparan sulfate proteoglycans can be shed via proteases into the extracellular matrix.2,3 Shed syndecan-1 remains biologically active and can promote tumor growth and metastasis.4 In addition to protease-mediated shedding of proteoglycans, the heparan sulfate chains of proteoglycans can be modified by extracellular endosulfatases that specifically remove 6-O sulfate groups.5 This structural change in heparan sulfate alters their capacity to regulate growth factor activities in a manner that can either promote or inhibit tumor growth.6 Heparan sulfate chains can also be altered by heparanase, an enzyme that cleaves heparan sulfate chains. This activity reduces the heparan sulfate content of the proteoglycan being attacked by the enzyme and also releases biologically active fragments of heparan sulfate that are 5 to 7 kDa in molecular size.7
Substantial data support the conclusion that heparanase promotes an aggressive phenotype in many tumor types. Much of this activity can be attributed to the fact that heparanase acts as a potent stimulator of tumor angiogenesis.7 This effect on angiogenesis probably occurs via several mechanisms. Heparanase enzyme activity has been associated with destruction of the basement membrane before cell invasion, an event that may enhance endothelial cell migration. Heparanase can also liberate growth factors that may be “stored” on the heparan sulfate chains present both at the cell surface and within the extracellular matrix. There is also evidence that the fragments of heparan sulfate generated by heparanase can bind to and facilitate growth factor activities that enhance angiogenesis.8 In addition, via nonenzymatic activity, heparanase can stimulate up-regulation of Akt signaling and vascular endothelial growth factor (VEGF) expression in tumor cells.9 Although there are data supporting all of these potential activities of heparanase, there are few data available to explain the precise molecular mechanism(s) of heparanase-mediated angiogenesis in tumors.
Using animal models and myeloma patient samples, we previously demonstrated that heparanase promotes angiogenesis, growth, and metastasis of myeloma cells to bone.10,11 We also discovered that heparanase stimulates enhanced expression and shedding of syndecan-1 from the surface of myeloma and breast cancer cells.12,13 Mechanistically, this occurs at least in part by heparanase-mediated stimulation of extracellular signal-regulated kinase (ERK) signaling that leads to an increase in matrix metalloproteinase–9 (MMP-9) expression.14 MMP-9 cleaves syndecan-1 core protein near the cell membrane, thereby releasing it from the cell surface as an intact extracellular domain bearing heparan sulfate chains. This enhanced shedding of syndecan-1 is important in myeloma because the proteoglycan remains biologically active and can stimulate myeloma growth and tumor progression in vivo.4 This parallels what is seen in some myeloma patients where heparanase activity and syndecan-1 shedding are up-regulated in the bone marrow and are associated with enhanced angiogenesis and poor prognosis.10,13,15
In the present study, using multiple myeloma tumor cells, we probed the mechanism whereby heparanase and shed syndecan-1 promote angiogenesis. Although we anticipated that the fragments of heparan sulfate generated by heparanase would stimulate angiogenesis, as was found in a skin cancer (melanoma) model,8 this was not the case in the multiple myeloma model. Surprisingly, we find that the heparanase-induced shedding of the intact syndecan-1 ectodomain is a key player in regulating endothelial cell invasion and angiogenesis. The shed syndecan-1 acts in concert with VEGF, which is also up-regulated by heparanase expression. Together, they drive angiogenesis. These results reveal an important new mechanistic pathway of heparanase action and demonstrate that the heparanase/syndecan-1 axis contributes significantly to tumor angiogenesis in myeloma and perhaps in other cancers.
Methods
Cell culture and transfection
RPMI-8226, MM.1S, and CAG myeloma cells were cultured in RPMI 1640 growth medium supplemented with 10% fetal bovine serum.16 CAG cells were transfected as previously described with empty vector or vector containing the cDNA for human heparanase to generate heparanase low (HPSE-low) and heparanase high (HPSE-high) cells, respectively.12 CAG cells were also transfected with a vector containing a mutated cDNA (M225 cells) that codes for enzymatically inactive heparanase.12 In the HPSE-low and HPSE-high cells, heparanase enzyme activity is associated with the cell and not detected in the conditioned medium (V. C. Ramani, unpublished observations, August 2008). Human umbilical vein endothelial cells (HUVECs) were obtained from Cambrex Bioscience and were cultured in endothelial cell basal medium-2 from Clonetics, supplemented with growth supplements and 2% fetal bovine serum. All experiments used HUVECs at passage 6 or less.
Matrigel invasion assay
The invasiveness of endothelial cells was analyzed using Biocoat Matrigel invasion chambers (BD Biosciences). The Matrigel invasion chamber consists of cell culture inserts containing an 8-μm pore size polyethylene membrane precoated with a thin layer of Matrigel basement membrane matrix. Endothelial cells (2 × 104) suspended in 500 μL of endothelial cell basal medium–2 media containing 1% fetal bovine serum were seeded in the upper compartment of chambers. A total of 750 μL of medium that had been conditioned by RPMI 8226 or MM.1S cells after treatment with recombinant human heparanase (100 ng/mL for 24 hours; kindly provided by Dr Israel Vlodavsky) was added to the lower well of the chamber. Complete RPMI 1640 medium not conditioned by cells but with added recombinant heparanase served as the control. In some experiments, conditioned media from CAG cells expressing low, high, or inactive heparanase was added to the lower compartment of the invasion chamber as the chemoattractant. Once cells were added to the upper chamber, they were incubated at 37°C and allowed to invade through the Matrigel barrier for 12 hours. After incubation, the noninvading cells were removed from the upper surface of the membrane using a cotton swab and invading cells on the underside of the filter were enumerated using an inverted microscope, after fixing and staining with Diff-Quick solution (IMEB Inc). Each assay was performed in triplicate. Data are expressed as the percentage of cell invasion with the migration stimulated by medium from HPSE-low cells set at 100%. For some experiments, the conditioned medium from CAG cells expressing high or low levels of heparanase was preincubated with a monoclonal antihuman VEGF Ab-3 antibody, which neutralizes the bioactivity of human VEGF (Thermo Scientific), or isotype specific control antibody. To determine the role of shed syndecan-1 in promoting endothelial invasion, cells were allowed to migrate in the presence of medium from HPSE-high cells pretreated with bacterial heparinase III (Hep III) or immunodepleted of syndecan-1. Syndecan-1 was immunodepleted from medium using 20 μg/mL of a polyclonal antihuman syndecan-1 ectodomain antibody17 bound to Protein-G Sepharose beads (GE Healthcare). Heparan sulfate chains were removed from syndecan-1 by addition of 5 mU/mL Hep III (Seikagaku) at 37°C for 1 hour. In some experiments, endothelial cells together with the ERK inhibitor PD98059 (50μM; Calbiochem), ERK inhibitor II negative control (Calbiochem), or 1μM synstatin (SSTN) peptide (active peptide SSTN92-119 or, as control, inactive peptide SSTN94-119).18 The active or control SSTN peptide was loaded into the upper compartment of the Matrigel invasion chamber. The invasion assay was also performed with conditioned medium from HPSE-high cells that was immunodepleted of syndecan-1 and to which was added recombinant VEGF (400 pg/mL; R&D Systems), syndecan-1 (500 ng/mL), or both. The amounts of VEGF and syndecan-1 used were equal to those amounts removed by immunoprecipitation of syndecan-1. Syndecan-1 produced by CAG cells was isolated and quantified as described.12,19
In assays where Matrigel was pretreated with medium before addition of cells, Matrigel-coated filters were incubated with medium conditioned by either HPSE-low or HPSE-high CAG myeloma cells or with medium from HPSE-high cells that was immunodepleted of syndecan-1 or treated with Hep III or with medium from cells expressing enzymatically inactive heparanase (M225). After 1 hour, the medium was removed and the invasion chamber was extensively washed with phosphate-buffered saline. Endothelial cells were seeded on the Matrigel; and after overnight incubation, cells that invaded through the Matrigel were counted.
Western blotting
ERK activation in endothelial cells was assessed by determining phosphorylation of ERK1/2 as described previously.14 Briefly, 2 × 105 endothelial cells were serum starved overnight followed by 15 minutes of incubation with 1 mL of conditioned medium from MM.1S or RPMI cells treated with recombinant human heparanase or with incubation with 1 mL of conditioned medium from HPSE-low or HPSE-high CAG myeloma cells with or without pretreatment with Hep III. At the designated time points, cells were washed and total cell lysates were subjected to immunoblotting with antibody against phospho-ERK (p-ERK; Cell Signaling). Equal loading was confirmed by probing the membrane with antibody to total ERK (t-ERK; Cell Signaling). Immunoreactive bands were detected using the ECL detection reagent (GE Healthcare). For some experiments, serum-starved endothelial cells were incubated with conditioned medium from HPSE-high myeloma cells in the presence of VEGF function–blocking antibody (Ab-3) or an isotype-matched control antibody. After 15 minutes, whole cell lysates were prepared and subjected to immunoblotting. To examine the potential role of shed syndecan-1 in activating ERK signaling, serum-starved HUVECs were treated for 15 minutes with medium conditioned by HPSE-high cells that was subjected to immunodepletion with control antibody or antibody to syndecan-1. For some experiments, endothelial cells were serum starved overnight and then stimulated for 15 minutes with syndecan-1–depleted conditioned medium from HPSE-high cells with or without addition of recombinant VEGF (400 pg/mL), purified syndecan-1 (500 ng/mL), or both. NIH ImageJ software was used to quantify the bands.
Quantification of VEGF
HPSE-low or HPSE-high CAG cells were plated at equal density in complete RPMI medium. After 48 hours, conditioned media were harvested and the level of VEGF was determined before and after immunodepletion of syndecan-1 from the medium. VEGF levels were quantified using an enzyme-linked immunosorbent assay (ELISA; Biosource). The standard curve was linear between 23 and 1500 pg/mL, and all samples were diluted to concentrations within that range.
Quantification of syndecan-1
Medium conditioned for 48 hours by CAG cells (106 cells/mL) was collected, and the levels of shed syndecan-1 present in the conditioned medium before and after incubating with Matrigel inserts for 1 hour was determined by ELISA as described.12
Heparan sulfate analysis
Conditioned medium harvested from HPSE-low and HPSE-high cells was adjusted to 80% ethanol and 1% sodium acetate and kept at 4°C overnight. The precipitated crude glycosaminoglycans and proteoglycans were recovered by centrifugation and desalted on an Amicon Ultra-4 Ultracel-3k (Millipore). An aliquot of the crude glycosaminoglycan and proteoglycan fraction was derivatized with 2-aminobenzamide (2AB; Nacalai Tesque),20 and excess 2AB was removed by extraction with chloroform.21 This labels reducing ends of the oligosaccharides, which are available only on the free glycosaminoglycan chains, not on the intact proteoglycans. The sample was then digested with a mixture of heparinase (EC 4.2.2.7) and heparitinase (EC 4.2.2.8; IBEX Technologies),22 and analyzed by anion-exchange high-performance liquid chromatography on a amine-bound silica PA-03 column (YMC). Disaccharide analysis of heparan sulfate was also carried out after labeling of a digest with a mixture of heparinase and heparitinase as previously reported.20
Aortic explant assay
Thoracic aortas were excised from male Sprague-Dawley rats and immediately placed into cold serum-free Dulbecco modified Eagle medium. Clotted blood inside the aorta was flushed with medium, and the periadventitial fibroadipose tissue was carefully removed. Aortas were then cut into cross-sectional pieces approximately 1.5 mm in length; 48-well plates were coated with 150 μL of Matrigel (BD Biosciences); after gelling at 37°C, the aortic explants were placed in the wells and sealed in place with an overlay of 150 μL of Matrigel. The wells were then overlaid with conditioned medium from myeloma cells along with 5 ng/mL recombinant VEGF. The cultures were maintained at 37°C for up to 10 days with medium changes every 2 days. Vascular sprouting from each explant was examined daily using an Olympus microscope, and digital images were obtained. Quantitative analysis of endothelial sprouting was performed using images from day 6. The number of sprouts was determined by counting the number of tube-like structures originating directly from the aortic ring (exclusive of branches) using National Institutes of Health ImageJ software.
Statistical analysis
Experiments were repeated a minimum of 3 times. Comparisons between 2 groups were analyzed by Student t test, and P less than .05 was considered statistically significant. Data are mean plus or minus SD.
Results
Heparanase enhances myeloma-mediated endothelial cell invasion
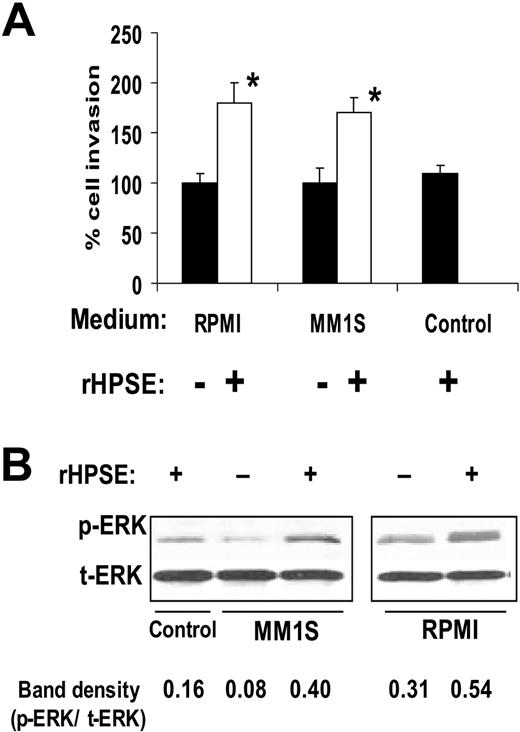
We used 3 cell lines and 2 different models to examine the effects of heparanase on myeloma-mediated endothelial cell invasion, a critical early step in the angiogenesis process. In the first model, we used recombinant human heparanase (rHPSE), which is taken up by the cells and remains biologically active.12,14,23 Twenty-four hours after addition of rHPSE to RPMI-8226 or MM.1S myeloma cell lines, the conditioned media from the cells were harvested and incubated with endothelial cells that had been plated on the upper surface of Matrigel-coated membranes in a Boyden chamber–type assay. Twelve hours later, the number of invading cells present on the underside of the filter was counted. Results show that medium from cells treated with rHPSE stimulated endothelial invasion significantly above the level of medium from cells not treated with rHPSE (Figure 1A). Conditioned medium from myeloma cells growing in the presence of rHPSE also enhanced levels of phospho-ERK in endothelial cells compared with cells growing in medium without addition of rHPSE (Figure 1B).
Heparanase promotes endothelial cell invasion and ERK phosphorylation. (A) Cells from myeloma cell lines RPMI 8226 or MM.1S were incubated for 24 hours with rHPSE and the conditioned medium collected. Endothelial cells were then placed in the upper chamber of Matrigel invasion chambers and conditioned medium from myeloma cells placed in the lower chamber. After overnight incubation, endothelial cells that invaded through the Matrigel were fixed, stained, and counted. The control was medium that was not conditioned by cells but with addition of rHPSE. Data are mean ± SD of 3 independent experiments. *P < .01 vs medium from cells not exposed to rHPSE. (B) Medium from myeloma cells was collected 24 hours after addition of rHPSE, added to endothelial cells in culture, and cells extracted and prepared for Western blotting for p-ERK and t-ERK. Bands were scanned and densities shown as ratios of p-ERK to t-ERK.
Heparanase promotes endothelial cell invasion and ERK phosphorylation. (A) Cells from myeloma cell lines RPMI 8226 or MM.1S were incubated for 24 hours with rHPSE and the conditioned medium collected. Endothelial cells were then placed in the upper chamber of Matrigel invasion chambers and conditioned medium from myeloma cells placed in the lower chamber. After overnight incubation, endothelial cells that invaded through the Matrigel were fixed, stained, and counted. The control was medium that was not conditioned by cells but with addition of rHPSE. Data are mean ± SD of 3 independent experiments. *P < .01 vs medium from cells not exposed to rHPSE. (B) Medium from myeloma cells was collected 24 hours after addition of rHPSE, added to endothelial cells in culture, and cells extracted and prepared for Western blotting for p-ERK and t-ERK. Bands were scanned and densities shown as ratios of p-ERK to t-ERK.
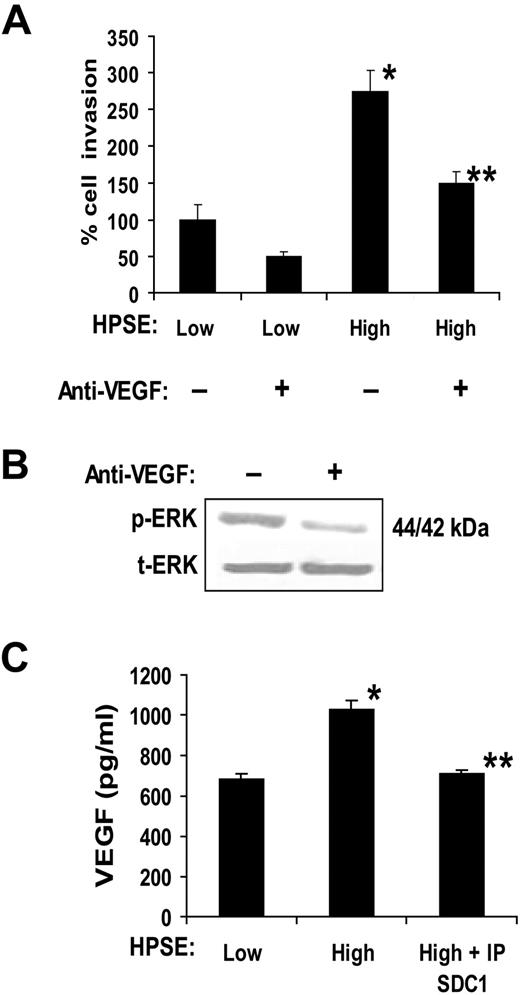
To confirm and extend the aforementioned findings, we used CAG myeloma cells that were transfected with the cDNA for human heparanase. These cells have been previously characterized and shown to have elevated levels of shed syndecan-1 in the HPSE-high cells compared with the control HPSE-low cells.12,14 When media conditioned by these cells were added to the lower well of the invasion chamber, endothelial cell invasion was potently stimulated by medium from HPSE-high cells compared with medium from HPSE-low cells (Figure 2A). Enhanced cell invasion was not the result of an increase in endothelial growth stimulated by the conditioned medium from HPSE-high cells because the number of viable endothelial cells was nearly identical 48 hours after treatment with either HPSE-high or HPSE-low medium (1.90 × 105 cells/mL and 1.95 × 105 cells/mL, respectively). Addition of a function-blocking anti-VEGF antibody (Ab-3) significantly inhibited the invasion-stimulatory activity present in the medium from the HPSE-high cells, thus pointing to VEGF as an important factor in driving endothelial invasion in this assay. Addition of the antibody also inhibited endothelial cell phosphorylation of ERK, thereby confirming the biologic activity of the VEGF and demonstrating that VEGF stimulates the MAPK/ERK pathway (Figure 2B). This is consistent with previous studies showing that VEGF enhances ERK phosphorylation, resulting in enhanced endothelial invasion and angiogenesis.24
Medium conditioned by heparanase high myeloma cells enhances endothelial invasion via activity of VEGF. (A) Endothelial cells were seeded on the top well of an invasion chamber coated with Matrigel and conditioned medium from either HPSE-low or HPSE-high cells was added to the lower well of the chamber in the presence or absence of a VEGF function blocking antibody. Data are mean ± SD from 3 independent experiments. *P < .001 vs HPSE-low without addition of anti-VEGF. **P < .01 vs HPSE-high. (B) VEGF stimulates ERK activation in endothelial cells. Serum-starved endothelial cells were incubated with conditioned medium from HPSE-high myeloma cells in the presence of VEGF function blocking antibody (Ab-3) or an isotype-matched control antibody. After 15 minutes, whole-cell lysates were prepared and subjected to immunoblotting for p-ERK and t-ERK. (C) VEGF levels are elevated in conditioned medium from HPSE-high cells and a portion of the VEGF associates with syndecan-1. HPSE-low or HPSE-high CAG cells were plated at equal density in complete RPMI medium. After 48 hours, conditioned media were harvested, and the level of VEGF was determined before and after immunodepletion of syndecan-1 (IP SDC1) from the medium (values represent means of triplicate determination ± SD). *P < .001 vs HPSE-low. **P < .001 vs HPSE-high.
Medium conditioned by heparanase high myeloma cells enhances endothelial invasion via activity of VEGF. (A) Endothelial cells were seeded on the top well of an invasion chamber coated with Matrigel and conditioned medium from either HPSE-low or HPSE-high cells was added to the lower well of the chamber in the presence or absence of a VEGF function blocking antibody. Data are mean ± SD from 3 independent experiments. *P < .001 vs HPSE-low without addition of anti-VEGF. **P < .01 vs HPSE-high. (B) VEGF stimulates ERK activation in endothelial cells. Serum-starved endothelial cells were incubated with conditioned medium from HPSE-high myeloma cells in the presence of VEGF function blocking antibody (Ab-3) or an isotype-matched control antibody. After 15 minutes, whole-cell lysates were prepared and subjected to immunoblotting for p-ERK and t-ERK. (C) VEGF levels are elevated in conditioned medium from HPSE-high cells and a portion of the VEGF associates with syndecan-1. HPSE-low or HPSE-high CAG cells were plated at equal density in complete RPMI medium. After 48 hours, conditioned media were harvested, and the level of VEGF was determined before and after immunodepletion of syndecan-1 (IP SDC1) from the medium (values represent means of triplicate determination ± SD). *P < .001 vs HPSE-low. **P < .001 vs HPSE-high.
Because previous work has shown that VEGF expression is up-regulated in tumor cells after enhancement of heparanase expression,9 we examined VEGF levels in the conditioned medium from the myeloma cells. ELISA results demonstrated that VEGF levels were higher in medium from HPSE-high myeloma cells than that from HPSE-low cells (Figure 2C). Moreover, immunodepletion of the heparan sulfate proteoglycan syndecan-1 from medium of HPSE-high cells removes a portion of the VEGF (Figure 2C). This partial binding of VEGF to syndecan-1 may reflect the inability of one of the isoforms of VEGF (VEGF121) to bind to heparan sulfate.25 Together, these results demonstrate that expression of heparanase by the myeloma cells enhances VEGF expression, leading to enhanced ERK signaling and endothelial invasion. In addition, a significant portion of the elevated VEGF in the conditioned medium is bound to syndecan-1.
Shed syndecan-1 participates in promoting endothelial invasion
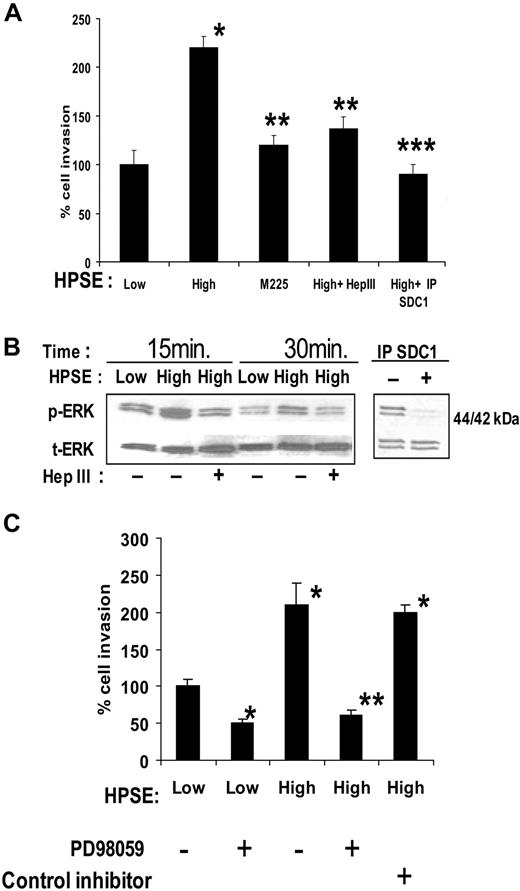
Heparan sulfate proteoglycans can play an important role in regulating VEGF activity because they bind to VEGF, potentiate binding of VEGF to its receptors on the endothelial surface,26 and can aid in establishing gradients of VEGF required to promote proper endothelial branching.27 Additional data suggest that heparan sulfate proteoglycans28 or exogenous heparin29 can bind to VEGF receptors themselves to facilitate signaling. Because heparanase expression by CAG myeloma cells enhances shedding of the syndecan-1 heparan sulfate proteoglycan12 and because shed syndecan-1 in the conditioned medium was binding VEGF (Figure 2C), we examined the role of shed syndecan-1 in promoting endothelial invasion. Pretreatment of medium from HPSE-high cells with the heparan sulfate-degrading enzyme bacterial Hep III removed the enhanced invasion-stimulatory activity of the medium (Figure 3A). It is important to note that Hep III extensively degrades heparan sulfate, whereas human heparanase only partially degrades heparan sulfate releasing 5- to 7-kDa fragments and leaving heparan sulfate proteoglycans intact but with reduced content of heparan sulfate.11,30
Shed syndecan-1 in conditioned medium of HPSE-high myeloma cells facilitates endothelial invasion and ERK phosphorylation. (A) Endothelial invasion is enhanced by shed syndecan-1. An equal number of endothelial cells were seeded on invasion chambers coated with Matrigel and cells allowed to migrate in the presence of medium conditioned by HPSE-low or HPSE-high CAG cells, or medium from HPSE-high cells pretreated with Hep III or IP SDC1 or with medium from cells expressing high levels of an enzymatically inactive form of heparanase (M225). After overnight incubation, cells that invaded through the Matrigel were fixed, stained, and counted. Data are mean ± SD of 3 independent experiments. *P < .01 vs HPSE-low. **P < .05 vs HPSE-high. ***P < .01 vs HPSE-high. (B left panel) Pretreatment of medium with Hep III diminishes ERK activation. Serum-deprived endothelial cells were treated with conditioned medium from HPSE-low or HPSE-high myeloma cells with or without pretreatment with Hep III. At the designated time points, cells were washed and total cell lysates were subjected to immunoblotting with antibodies against p-ERK or t-ERK. (Right panel) ERK signaling is lost after depletion of syndecan-1 from conditioned medium. Medium conditioned by HPSE-high cells was subjected to immunodepletion with control antibody or antibody to syndecan-1. Serum-deprived endothelial cells were treated with this medium for 15 minutes, lysed, and subjected to immunoblotting with antibodies against p-ERK or t-ERK. (C) ERK activation stimulates invasion of endothelial cells. Equal number of endothelial cells along with dimethyl sulfoxide as control, ERK inhibitor PD98059 (50μM), or ERK inhibitor II negative control (50μM) were loaded into the upper compartment of the Matrigel invasion chamber and medium from HPSE-low or HPSE-high cells added to the lower compartment. After overnight incubation, cells that invaded through the Matrigel were fixed, stained, and counted. Data represent mean ± SD of 3 independent experiments. *P < .01 vs HPSE-low without addition of PD98059. **P < .01 vs HPSE-high without addition of PD98059.
Shed syndecan-1 in conditioned medium of HPSE-high myeloma cells facilitates endothelial invasion and ERK phosphorylation. (A) Endothelial invasion is enhanced by shed syndecan-1. An equal number of endothelial cells were seeded on invasion chambers coated with Matrigel and cells allowed to migrate in the presence of medium conditioned by HPSE-low or HPSE-high CAG cells, or medium from HPSE-high cells pretreated with Hep III or IP SDC1 or with medium from cells expressing high levels of an enzymatically inactive form of heparanase (M225). After overnight incubation, cells that invaded through the Matrigel were fixed, stained, and counted. Data are mean ± SD of 3 independent experiments. *P < .01 vs HPSE-low. **P < .05 vs HPSE-high. ***P < .01 vs HPSE-high. (B left panel) Pretreatment of medium with Hep III diminishes ERK activation. Serum-deprived endothelial cells were treated with conditioned medium from HPSE-low or HPSE-high myeloma cells with or without pretreatment with Hep III. At the designated time points, cells were washed and total cell lysates were subjected to immunoblotting with antibodies against p-ERK or t-ERK. (Right panel) ERK signaling is lost after depletion of syndecan-1 from conditioned medium. Medium conditioned by HPSE-high cells was subjected to immunodepletion with control antibody or antibody to syndecan-1. Serum-deprived endothelial cells were treated with this medium for 15 minutes, lysed, and subjected to immunoblotting with antibodies against p-ERK or t-ERK. (C) ERK activation stimulates invasion of endothelial cells. Equal number of endothelial cells along with dimethyl sulfoxide as control, ERK inhibitor PD98059 (50μM), or ERK inhibitor II negative control (50μM) were loaded into the upper compartment of the Matrigel invasion chamber and medium from HPSE-low or HPSE-high cells added to the lower compartment. After overnight incubation, cells that invaded through the Matrigel were fixed, stained, and counted. Data represent mean ± SD of 3 independent experiments. *P < .01 vs HPSE-low without addition of PD98059. **P < .01 vs HPSE-high without addition of PD98059.
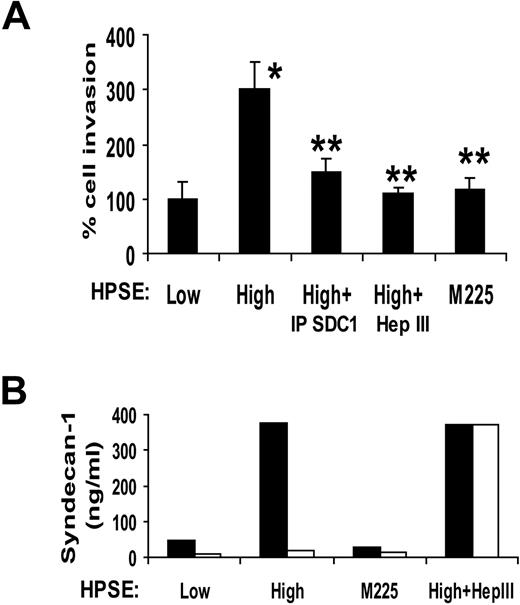
Immunodepletion of shed syndecan-1 from the medium of HPSE-high cells also diminished the stimulation of endothelial invasion, thus confirming the role of the proteoglycan in promoting invasion (Figure 3A). Removal of syndecan-1 from medium conditioned by HPSE-low cells had no significant effect on the invasion of endothelial cells (A.P., unpublished observation, December 2008), suggesting that the basal level of endothelial invasion is independent of shed syndecan-1. It is probable that the basal level of endothelial invasion seen in cells treated with medium from HPSE-low cells is facilitated by syndecan-1, syndecan-2, and/or syndecan-4, which are known to be present on the endothelial cell surface.18,31 Interestingly, medium from cells expressing a mutated form of heparanase (M225) that lacks heparan sulfate–degrading enzyme activity failed to enhance endothelial invasion (Figure 3A). The CAG-M225 cells expressing this enzymatically inactive heparanase do not exhibit elevated levels of shed syndecan-1 compared with cells expressing enzymatically active heparanase.12 Together, these results indicate that the stimulatory effect of the HPSE-high cells on endothelial invasion requires the expression of the active heparanase enzyme and shed syndecan-1 with attached heparan sulfate chains.
Analysis of ERK phosphorylation in endothelial cells after their incubation with conditioned medium from HPSE-high cells revealed an enhanced level of phosphorylation compared with medium from HPSE-low cells (Figure 3B). This enhanced phosphorylation of ERK was blocked by pretreating medium from HPSE-high cells with Hep III or by removal of syndecan-1 from the medium by immunoprecipitation (Figure 3B). Thus, the enhanced signaling and invasion of cells exposed to medium from HPSE-high cells can be blocked by interfering with either VEGF (Figure 2) or shed syndecan-1 (Figure 3). Treatment of the endothelial cells with the ERK inhibitor PD98059 greatly diminished endothelial cell invasion, reflecting the requirement for ERK activation to initiate invasion of these cells (Figure 3C).
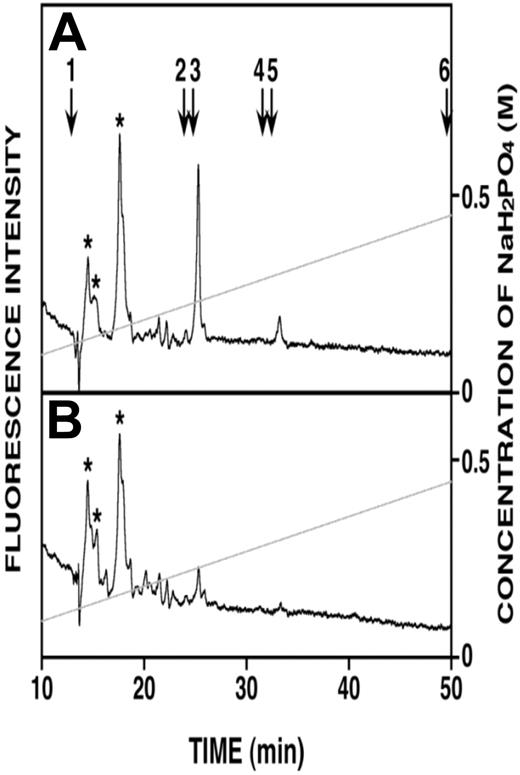
Because heparanase activity generates fragments of heparan sulfate that are reported to retain biologic activity,7 it was possible that these fragments were contributing to endothelial invasion. However, immunoprecipitation of syndecan-1 captures the intact core protein having heparan sulfate chains that are trimmed by heparanase, and the fragments of heparan sulfate released by the heparanase remain in the conditioned medium. Our finding that removal of syndecan-1 from medium depletes essentially all of the enhanced invasion of the endothelial cells (Figure 3A) indicates that the remaining fragments of heparan sulfate have no effect on stimulation of endothelial invasion. To examine this directly, heparan sulfate fragments in the medium conditioned by the HPSE-low and HPSE-high cells were analyzed. Figure 4 shows that no heparan sulfate fragments were detected in medium from HPSE-low cells; and although present in the medium from HPSE-high cells, the levels of heparan sulfate fragments were very low, representing only 22 pmol of heparan sulfate fragments compared with the total heparan sulfate present in the medium (70 nmol as disaccharides; data not shown). Our previous work shows that most of the heparanase is located within the myeloma cells,10,11 and not extracellularly. Therefore, most trimming of the heparan sulfate chains occurs intracellularly where the heparan sulfate fragments are then degraded. Thus, almost all of the heparan sulfate in the conditioned medium (extracellular compartment) is present as a component of proteoglycans, not free heparan sulfate chains. In myeloma, the predominant heparan sulfate proteoglycan is syndecan-1, and we have shown that in CAG cells, there are little, if any, other heparan sulfate proteoglycans present.32 The low abundance of heparan sulfate in the medium from HPSE-high cells further supports the conclusion that it is the heparanase-digested syndecan-1, not heparan sulfate fragments, that enhance endothelial invasion.
Very low levels of heparan sulfate fragments are present in medium from HPSE-high cells. (A) Anion-exchange high-performance liquid chromatography of heparan sulfate fragments eluting at 25 minutes (monosulfated oligosaccharides) and 33 minutes (disulfated oligosaccharides) were detected in the medium conditioned by HPSE-high cells. (B) Heparan sulfate fragments were not detected in medium conditioned by HPSE-low cells. Elution positions of the 2AB-derivatives of standard unsaturated heparan sulfate disaccharides are indicated by the numbered arrows in panel A: 1, ΔHexA-GlcNAc; 2, ΔHexA-GlcNAc(6-O-sulfate); 3, ΔHexA-GlcN(N-sulfate); 4, ΔHexA-GlcN(N, 6-O-disulfate); 5, ΔHexA(2-O-sulfate)-GlcN(N-sulfate); 6, ΔHexA(2-O-sulfate)-GlcN(N, 6-O-disulfate). *Peaks representing unknown substances derived from the sample preparation.
Very low levels of heparan sulfate fragments are present in medium from HPSE-high cells. (A) Anion-exchange high-performance liquid chromatography of heparan sulfate fragments eluting at 25 minutes (monosulfated oligosaccharides) and 33 minutes (disulfated oligosaccharides) were detected in the medium conditioned by HPSE-high cells. (B) Heparan sulfate fragments were not detected in medium conditioned by HPSE-low cells. Elution positions of the 2AB-derivatives of standard unsaturated heparan sulfate disaccharides are indicated by the numbered arrows in panel A: 1, ΔHexA-GlcNAc; 2, ΔHexA-GlcNAc(6-O-sulfate); 3, ΔHexA-GlcN(N-sulfate); 4, ΔHexA-GlcN(N, 6-O-disulfate); 5, ΔHexA(2-O-sulfate)-GlcN(N-sulfate); 6, ΔHexA(2-O-sulfate)-GlcN(N, 6-O-disulfate). *Peaks representing unknown substances derived from the sample preparation.
VEGF binds shed syndecan-1 and together they stimulate endothelial invasion
Having established that both VEGF and shed syndecan-1 were contributing to enhancing endothelial cell invasion, we next examined more directly the effects of VEGF and syndecan-1 on endothelial invasion. To do this, medium from HPSE-high cells was immunodepleted of syndecan-1 (which also depleted VEGF, Figure 2C), and recombinant VEGF and/or purified syndecan-1 were added back to the medium and the effects on endothelial invasion measured. With addition of the same amount of VEGF that was removed by depletion of syndecan-1, some of the invasive activity of the medium was restored, whereas addition of syndecan-1 alone had no effect (Figure 5A). Addition of both recombinant VEGF and purified syndecan-1 to the syndecan-1-depleted medium fully restores the endothelial invasive activity of the HPSE-high medium. Similarly, ERK stimulation is maximal when both syndecan-1 and VEGF were added together to the medium depleted of syndecan-1 (Figure 5B). The necessity for both VEGF and syndecan-1 acting together to stimulate invasion is also demonstrated by the finding that Hep III treatment of conditioned medium abolished the stimulation of endothelial invasion (Figure 3A) even though VEGF remains intact in the medium after Hep III treatment.
Shed syndecan-1 and VEGF act together to stimulate ERK activation and endothelial invasion. (A) Syndecan-1 enhances VEGF-mediated invasion of endothelial cells. An endothelial invasion assay was performed with conditioned medium from HPSE-high cells that was IP SDC1 and to which was added recombinant VEGF (400 pg/mL), syndecan-1 (500 ng/mL), or both. The amounts of VEGF and syndecan-1 used were equal to those amounts removed by immunoprecipitation of syndecan-1. Data are mean ± SD from 3 independent experiments. *P < .001 vs HPSE-low. **P < .001 vs HPSE-high. ***P < .01 vs IP SCD1 with no exogenous VEGF or syndecan-1. ****P < .05 vs exogenous VEGF alone. (B) Syndecan-1 and VEGF in combination maximally stimulate ERK signaling. Endothelial cells were serum starved overnight and then stimulated with syndecan-1 depleted conditioned medium from HPSE-high cells with or without addition of recombinant VEGF (400 pg/mL), purified syndecan-1 (500 ng/mL), or both. Aliquots of cell extracts that contained equivalent amounts of total protein were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and then immunoblotted using antibody specific for p-ERK or t-ERK. Bands were scanned and densities are shown as ratios of p-ERK to t-ERK.
Shed syndecan-1 and VEGF act together to stimulate ERK activation and endothelial invasion. (A) Syndecan-1 enhances VEGF-mediated invasion of endothelial cells. An endothelial invasion assay was performed with conditioned medium from HPSE-high cells that was IP SDC1 and to which was added recombinant VEGF (400 pg/mL), syndecan-1 (500 ng/mL), or both. The amounts of VEGF and syndecan-1 used were equal to those amounts removed by immunoprecipitation of syndecan-1. Data are mean ± SD from 3 independent experiments. *P < .001 vs HPSE-low. **P < .001 vs HPSE-high. ***P < .01 vs IP SCD1 with no exogenous VEGF or syndecan-1. ****P < .05 vs exogenous VEGF alone. (B) Syndecan-1 and VEGF in combination maximally stimulate ERK signaling. Endothelial cells were serum starved overnight and then stimulated with syndecan-1 depleted conditioned medium from HPSE-high cells with or without addition of recombinant VEGF (400 pg/mL), purified syndecan-1 (500 ng/mL), or both. Aliquots of cell extracts that contained equivalent amounts of total protein were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and then immunoblotted using antibody specific for p-ERK or t-ERK. Bands were scanned and densities are shown as ratios of p-ERK to t-ERK.
The syndecan-1 core protein participates in promoting endothelial invasion
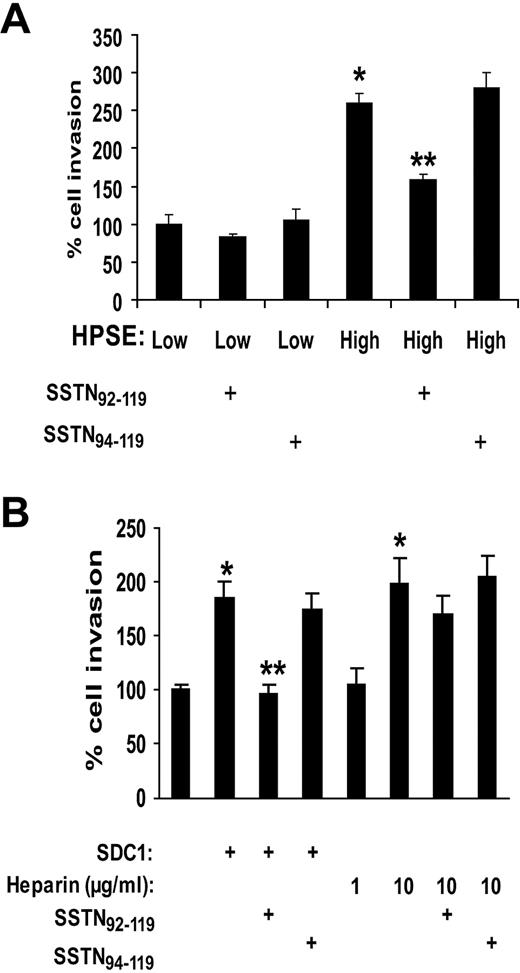
Although these results indicate that heparan sulfate chains of shed syndecan-1 are critical for enhancing endothelial invasion, it was recently demonstrated that a region of the syndecan-1 core protein ectodomain forms a complex with αvβ3 and αvβ5 integrins, causing activation of the integrin that mediates cell spreading and invasion.18 In that study, SSTN, a peptide mimic of the syndecan-1 ectodomain that blocks integrin activation, was shown to block angiogenesis in aortic explants and in mouse mammary epithelial tumors in vivo. Thus, we speculated that syndecan-1 shed by myeloma cells might be activating integrins on endothelial cells and contributing to their invasive phenotype. To test this, we added exogenous SSTN to our endothelial invasion assay. Results show that SSTN blocks the increased stimulation of endothelial invasion by medium from HPSE-high cells and has no effect on invasion mediated by medium from the HPSE-low cells (Figure 6A). Similarly, active SSTN peptide inhibits endothelial invasion in the presence of exogenous VEGF and syndecan-1 added to medium that had been previously immunodepleted of syndecan-1 (Figure 6B). If heparin is substituted for intact syndecan-1, it cannot restore invasive activity unless it is added at a much higher concentration than is present in the conditioned medium from HPSE-high cells. This effect of excess heparin cannot be blocked by SSTN, indicating that, when heparin is present at high concentrations, the activity of the syndecan-1 core protein is not required for promoting invasion. This further confirms the importance of the syndecan-1 core protein in promoting endothelial invasion in physiologic situations where the concentrations of heparan sulfate within the microenvironment are not artificially high. Together, the demonstration that SSTN inhibits endothelial cell invasion indicates that shed syndecan-1 plays a dual role in angiogenesis via activity of the core protein and binding of VEGF to heparan sulfate.
Stimulation of invasion of endothelial cells is blocked by SSTN, a peptide that inhibits syndecan-1–mediated activation of integrins. (A) Active SSTN peptide inhibits invasion of endothelial cells. The Matrigel invasion chamber assay was carried out using conditioned medium from HPSE-high or HPSE-low cells with addition of 1μM active SSTN92-119, or inactive (control) SSTN94-119 peptide. *P < .05 vs HPSE-low. **P < .05 vs HPSE-high in the presence of SSTN94-119. (B) Medium from HPSE-high cells was depleted of syndecan-1 (which also depletes VEGF). Addition of VEGF (which was added to all wells) and syndecan-1 to the medium restored the invasive phenotype, but this was blocked by addition of active SSTN. Addition of heparin (rather than syndecan-1) at a concentration similar to that of heparan sulfate present in the exogenous syndecan-1 (∼ 1 μg/mL) did not restore the highly invasive phenotype, whereas addition of 10 μg/mL heparin enhanced invasion as well exogenous syndecan-1. SSTN peptide failed to block cell invasion in the presence of the high (10 μg/mL) level of heparin.
Stimulation of invasion of endothelial cells is blocked by SSTN, a peptide that inhibits syndecan-1–mediated activation of integrins. (A) Active SSTN peptide inhibits invasion of endothelial cells. The Matrigel invasion chamber assay was carried out using conditioned medium from HPSE-high or HPSE-low cells with addition of 1μM active SSTN92-119, or inactive (control) SSTN94-119 peptide. *P < .05 vs HPSE-low. **P < .05 vs HPSE-high in the presence of SSTN94-119. (B) Medium from HPSE-high cells was depleted of syndecan-1 (which also depletes VEGF). Addition of VEGF (which was added to all wells) and syndecan-1 to the medium restored the invasive phenotype, but this was blocked by addition of active SSTN. Addition of heparin (rather than syndecan-1) at a concentration similar to that of heparan sulfate present in the exogenous syndecan-1 (∼ 1 μg/mL) did not restore the highly invasive phenotype, whereas addition of 10 μg/mL heparin enhanced invasion as well exogenous syndecan-1. SSTN peptide failed to block cell invasion in the presence of the high (10 μg/mL) level of heparin.
Shed syndecan-1 binds to Matrigel to promote endothelial invasion
Before addition of endothelial cells, Matrigel-coated invasion chambers were incubated with conditioned medium from HPSE-low or HPSE-high myeloma cells or medium from cells expressing enzymatically inactive heparanase. The conditioned medium was then removed, endothelial cells added, and invasion was monitored. Clearly, the invasion-stimulatory activity was transferred from the medium to the Matrigel during the preincubation period (Figure 7A). The activity transferred from the medium of HPSE-high cells is greater than that from medium conditioned by HPSE-low cells or from cells expressing mutated heparanase. Moreover, immunodepletion of syndecan-1 from the medium before incubation with the wells diminishes the transfer of invasive activity to the Matrigel-coated invasion chamber. Treatment of the conditioned medium with Hep III before incubation with the Matrigel also diminished the invasion-stimulatory activity of the conditioned medium. Results with Hep III again indicate that the VEGF in the medium cannot stimulate endothelial invasion in the absence of intact shed syndecan-1. Quantification of syndecan-1 in the conditioned medium before and after incubation with the Matrigel confirms that the soluble syndecan-1 binds to the Matrigel-coated filters and that this is dependent on the syndecan-1 heparan sulfate chains (Figure 7B). Taken together, the data indicate that the shed syndecan-1 with bound VEGF attaches to the Matrigel and promotes endothelial invasion. Shed syndecan-1 may potentiate VEGF activity by several mechanisms. These could include aiding in concentrating VEGF within the extracellular matrix adjacent to the endothelial cells and/or presenting the VEGF to its receptor(s) on the endothelial cell surface. In addition, binding of VEGF to the syndecan-1 heparan sulfate may protect VEGF from proteolytic degradation.
Shed syndecan-1 binds to the extracellular matrix to promote invasion and angiogenesis. (A) Matrigel invasion chambers were incubated with medium conditioned by either HPSE-low or HPSE-high CAG myeloma cells or with medium from HPSE-high cells that was immunodepleted of syndecan-1 or treated with Hep III or with medium from cells expressing enzymatically inactive heparanase (M225). After 1 hour, the medium was removed, endothelial cells were seeded on the Matrigel, and after overnight incubation, cells that invaded through the Matrigel were counted. Data are mean ± SD from 3 independent experiments. *P < .05 vs HPSE-low. **P < .05 vs HPSE-high. (B) The level of syndecan-1 present in the conditioned medium before (■) and after (□) incubation with the Matrigel was determined by ELISA. Treatment of conditioned medium with Hep III abolished binding of syndecan-1 to the Matrigel.
Shed syndecan-1 binds to the extracellular matrix to promote invasion and angiogenesis. (A) Matrigel invasion chambers were incubated with medium conditioned by either HPSE-low or HPSE-high CAG myeloma cells or with medium from HPSE-high cells that was immunodepleted of syndecan-1 or treated with Hep III or with medium from cells expressing enzymatically inactive heparanase (M225). After 1 hour, the medium was removed, endothelial cells were seeded on the Matrigel, and after overnight incubation, cells that invaded through the Matrigel were counted. Data are mean ± SD from 3 independent experiments. *P < .05 vs HPSE-low. **P < .05 vs HPSE-high. (B) The level of syndecan-1 present in the conditioned medium before (■) and after (□) incubation with the Matrigel was determined by ELISA. Treatment of conditioned medium with Hep III abolished binding of syndecan-1 to the Matrigel.
Heparanase enhanced shedding of syndecan-1 promotes angiogenesis in aortic organ cultures
Because our findings indicated that shed syndecan-1 facilitates VEGF-mediated signaling and cellular responses in cultured endothelial cells, we examined the potential role of shed syndecan-1 in promoting angiogenesis using rat aortic organ cultures. This model recapitulates the entire process of angiogenesis and is known to be driven, at least in part, via the VEGF pathway. Indeed, successful microvessel outgrowth in this assay requires exogenous VEGF. Addition of conditioned medium from HPSE-low or HPSE-high cells alone did not stimulate microvessel outgrowth. However, after addition of exogenous VEGF, microvessel outgrowth from the aortic explants was significantly greater in explants receiving medium from HPSE-high cells than that from addition of medium from HPSE-low cells (Figure 8). As with the endothelial invasion assays, depletion of syndecan-1 from the HPSE-high conditioned medium greatly diminished the stimulation of angiogenesis. Moreover, addition of VEGF and syndecan-1 to medium conditioned by HPSE-low cells enhanced angiogenesis. Together, these data confirm that shed syndecan-1 plays a key role in stimulating angiogenesis.
Shed syndecan-1 in conditioned medium from HPSE-high cells promotes angiogenesis in an aortic organ culture. (A) Segments of rat aorta were placed within Matrigel and cultured in the presence of medium conditioned by either HPSE-low or HPSE-high CAG cells, with medium from HPSE-low cells with addition of exogenous syndecan-1 (500 ng/mL) or with medium from HPSE-high cells that was immunodepleted of syndecan-1 (exogenous VEGF was added to all wells). Note that more microvessels grow out from the explants treated with conditioned media from HPSE-high cells versus HPSE-low cells. Addition of exogenous syndecan-1 to HPSE-low condition medium enhanced microvessel density, and immunodepletion of syndecan-1 from the conditioned medium of HPSE- high cells reduced the microvessel formation. Bar represents 0.2 mm. (B) Quantitative analysis of endothelial sprouting was performed using images from day 6, and sprout number was quantified using NIH ImageJ software. Data shown are from 2 separate experiments, each of which contained duplicate wells for each condition. *P < .01 vs HPSE-low. **P < .01 vs HPSE-high.
Shed syndecan-1 in conditioned medium from HPSE-high cells promotes angiogenesis in an aortic organ culture. (A) Segments of rat aorta were placed within Matrigel and cultured in the presence of medium conditioned by either HPSE-low or HPSE-high CAG cells, with medium from HPSE-low cells with addition of exogenous syndecan-1 (500 ng/mL) or with medium from HPSE-high cells that was immunodepleted of syndecan-1 (exogenous VEGF was added to all wells). Note that more microvessels grow out from the explants treated with conditioned media from HPSE-high cells versus HPSE-low cells. Addition of exogenous syndecan-1 to HPSE-low condition medium enhanced microvessel density, and immunodepletion of syndecan-1 from the conditioned medium of HPSE- high cells reduced the microvessel formation. Bar represents 0.2 mm. (B) Quantitative analysis of endothelial sprouting was performed using images from day 6, and sprout number was quantified using NIH ImageJ software. Data shown are from 2 separate experiments, each of which contained duplicate wells for each condition. *P < .01 vs HPSE-low. **P < .01 vs HPSE-high.
Discussion
This work reveals a novel mechanistic pathway demonstrating how heparanase mediates endothelial invasion, a key early step in angiogenesis. We have previously demonstrated the mechanism whereby heparanase up-regulates syndecan-1 shedding. This occurs via heparanase stimulation of ERK phosphorylation, with resulting up-regulation of MMP-9, which then acts as a sheddase of syndecan-1.14 The heparanase-mediated up-regulation of VEGF in these myeloma cells, together with the enhanced level of syndecan-1 shedding, stimulates endothelial invasion and angiogenesis. Importantly, to get maximal stimulation of angiogenesis, the presence of both syndecan-1 and VEGF together is required. Moreover, we demonstrated that, when released as soluble factors, the syndecan-1 with bound VEGF can bind to extracellular matrix and subsequently stimulate endothelial cell invasion. This provides an important mechanism whereby myeloma cells can release these factors that then become concentrated within the tumor microenvironment and promote angiogenesis.
Previous work has demonstrated that fragments of heparan sulfate generated by heparanase cleavage can modulate the activity of growth factors, such as FGF2 and VEGF, and can also stimulate melanoma angiogenesis in vivo.8,33 Thus, it is interesting that, in the models we used in the present work (invasion chamber assay and aortic explants), we find that it is not the heparan sulfate fragments released by heparanase that stimulate endothelial invasion; rather, it is the intact syndecan-1. This finding indicates that stimulation of invasion is the result of either the enhanced level of syndecan-1 in the medium of heparanase transfected cells or, possibly, changes in heparan sulfate structure that result from the presence of increased levels of heparanase. It has been demonstrated that, in heparanase transgenic mice, the heparan sulfate chains produced have higher levels of sulfation than is present in control mice, and this higher level of sulfation enhances heparan sulfate interactions with growth factors.34 Moreover, heparanase trimming of heparan sulfate chains could unmask functional domains within the core protein or unveil cryptic sites along the chain that have unique biologic activities.35 However, as related to our findings, it does not seem probable that modifications to syndecan-1 heparan sulfate resulting from heparanase expression are causing the enhanced endothelial invasion. This is because, after immunoprecipitation of syndecan-1 from conditioned medium and the resulting loss of the enhanced invasive activity, we were able to restore the invasive activity by addition of syndecan-1 purified from medium of cells that did not express high levels of heparanase. This indicates that the enhanced ability of medium conditioned by heparanase-high cells to stimulate endothelial invasion is the result of the increase in the amount of syndecan-1 present.
Although heparan sulfate on the surface of endothelial cells is known to play a role in promoting angiogenesis,31 it has been shown that heparan sulfate proteoglycan present on cells surrounding endothelium can also contribute to angiogenesis. For example, syndecan-1 expression by stromal cells stimulates angiogenesis of breast carcinomas36 and heparan sulfate on the surface of perivascular smooth muscle cells can potentiate VEGF signaling on adjacent endothelial cells.37 It has also been shown that heparan sulfate aids in establishing gradients of VEGF required to promote proper endothelial branching.27 Our findings indicate that shed syndecan-1 with bound VEGF released from the tumor cell infiltrates and binds to the extracellular matrix where it promotes endothelial invasion and angiogenesis. Binding to the extracellular matrix in the model used here is dependent on heparan sulfate because pretreatment with Hep III abolishes binding. The heparan sulfate has the potential to bind many macromolecules within the matrix but probably occurs in our model via binding to laminin, a major component of Matrigel.
Our findings indicate that the process of syndecan-1 shedding can extend the range of proteoglycan function beyond that of cell-cell contacts within the tumor microenvironment. This capability could enhance tumor growth by “jump-starting” angiogenesis at sites where cell surface proteoglycan and/or VEGF expression may not yet be at levels sufficient for initiating angiogenesis. More broadly, syndecan-1 with bound angiogenic factors released by tumor cells may also enter the circulation and nurture angiogenesis at sites distal to the tumor, perhaps helping to establish premetastatic niches.38 Although our data suggest that the majority of angiogenic activity in the model systems used here can be traced to VEGF (Figure 2A), it is possible that syndecan-1 interactions with other myeloma-derived angiogenic factors (eg, hepatocyte growth factor) also contribute to the robust angiogenic response of this cancer.
Central to this process of shed syndecan-1 enhancing tumor angiogenesis is the up-regulation of syndecan-1 shedding by heparanase. Based on our current and previous findings, we propose a model whereby heparanase stimulates up-regulation of MMP-9 expression, leading to enhanced syndecan-1 shedding.14 The shed syndecan-1 then exerts dual proangiogenic effects by presenting VEGF to endothelial cells and by activating integrins on the endothelial cell surface. This may be particularly important in regard to αvβ3 integrin, which is known to be a key regulator of endothelial activation and angiogenesis.39 Indeed, activation of the αvβ3 integrin and VEGFR2 on endothelial cells has been shown to be synergistic; that is, VEGF enhances the activation of this integrin and integrin-mediated migration of the endothelial cells, and the αvβ3 integrin in turn is critical for the VEGF-mediated activation of VEGFR2.40 It is intriguing to speculate that syndecan-1, which engages the αvβ3 integrin on endothelial cells and is essential for its activation, is also playing a secondary role in providing VEGF to enhance this activation mechanism. VEGF bound to syndecan-1, rather than to other HSPGs in the matrix, would be most effective at integrin activation as it would be directly supplied by syndecan-1 to VEGFR2 complexed with the integrin. This is also consistent with our finding that heparin can substitute for syndecan-1 in promoting endothelial invasion only when the heparin is added at a high concentration (Figure 6B).
Our model is also supported by a recent report demonstrating that angiogenesis is stimulated in vitro by addition of exogenous MMP-9 to HT29 colorectal carcinoma spheroids. In this system, the exogenous MMP-9 released heparan sulfate proteoglycans with bound VEGF, leading to an enhanced angiogenic response.41 We have demonstrated that heparanase expression leads to increased MMP-9 production and syndecan-1 shedding in myeloma cancer cells.14 Thus, it is reasonable that a similar mechanism might also be at play in colorectal cancers where heparanase expression is elevated.42 This raises the possibility that the heparanase-driven mechanism we describe here using myeloma cells may be broadly applicable to several tumor types in which heparanase is up-regulated, leading to stimulation of angiogenesis and enhanced tumor progression.
Our findings are relevant to the progression of disease in myeloma patients because it has been reported that high levels of heparanase or shed syndecan-1 are indicators of poor prognosis in myeloma.13,43 Moreover, elevated expression of heparanase or syndecan-1 in patients is associated with high levels of tumor-associated angiogenesis.10,44 Using in vivo models of myeloma, we have previously demonstrated that heparanase and shed syndecan-1 can enhance myeloma tumor growth, angiogenesis, and metastasis.4,10,11 The present work adds important mechanistic insight into understanding tumor angiogenesis and provides further explanation as to how heparanase and syndecan-1 participate in driving the aggressive phenotype in myeloma.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Joseph Ritchie for help with VEGF ELISA assays and aortic explant experiments, Dr Steven T. Rosen (Northwestern University) for providing MM.1S cells, and Dr Israel Vlodavsky (Technion, Haifa, Israel) for providing recombinant heparanase.
This work was supported by the National Institutes of Health (grants CA103054 and CA135075, R.D.S.; CA118839, CA139873, and CA109010, A.C.R.; and Human Science Program grant RGP18/2005, K.S.).
National Institutes of Health
Authorship
Contribution: A.P., T.U., and R.D.S. designed the study; A.P. and R.D.S. wrote the paper; A.P and T.U. performed the research and analyzed the data; A.C.R. provided synstatin and guidance on its use, and provided critical discussions and reading of the manuscript; and F.K., S.Y., and K.S. designed and performed analysis of heparan sulfate fragments.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current address of T.U. is Department of Biochemistry, Kagawa University School of Medicine, 1750-1 Ikenobe, Miki, Kagawa, 761-0793, Japan.
Correspondence: Ralph D. Sanderson, Department of Pathology, University of Alabama at Birmingham, 1530 Third Ave South, SHEL 814, Birmingham, AL 35294; e-mail: sanderson@uab.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal