Previous case-control studies showed that genetic variation in the fibrinogen γ gene (FGG) increased the risk for deep vein thrombosis (VT) in adults. We investigated the association between the fibrinogen α (FGA) and FGG haplotypes, the factor VLeiden-mutation, and pediatric VT and thromboembolic stroke (TS) in 2 independent study samples. Association analysis revealed that the FGA-H1 and FGG-H2 haplotypes were significantly overtransmitted to VT patients (FGA-H1, P = .05; FGG: H2, P = .032). In contrast, the FGG-H3 haplotype was undertransmitted (P = .022). In an independent study sample, FGA-H1 (P = .008) and FGG-H2 (P = .05) were significantly associated with TS. The association of FGA and FGG haplotypes with VT was more pronounced in FVLeiden-negative families (FGA-H1, P = .001; FGG-H2, P = .001), indicating a genetic interaction between both risk factors. The risk-conferring FGG-H2 and the protective FGG-H3 haplotypes correlated with low (FGG-H2) and high (FGG-H3) levels of the γ′ chain variant, respectively. These results provide independent and novel evidence that FGA-H1 and FGG-H2 variants are associated with an increased risk of VT and TS in children. The observed negative correlation of genetic VT risk with the plasma levels of the fibrinogen γ′ variant suggests that FGG-H2 and -H3 haplotypes modify thrombosis risk by controlling the level of this FGG splice isoform.
Introduction
Population-based association studies in adults have provided sound evidence for the association of fibrinogen levels with arterial disease, and for the association of fibrinogen gene variations with fibrinogen levels. For example, abnormalities of fibrinogen levels have been reported to affect risk for deep vein thrombosis (VT), particularly in the elderly.1,2 However, evidence for the association of disease and fibrinogen gene polymorphisms thought to affect fibrinogen levels is weak.3,4 One of the mechanisms by which changes in the fibrin network may contribute to an increased VT risk may hinge on the composition of circulating total fibrinogen levels, particularly the fraction of fibrinogen γ′ levels, which in part is influenced by genetic variants residing in the fibrinogen gamma (FGG) gene located on the long arm of human chromosome 4 (4q28). A recent study investigating the role of genetic variants in predisposing to VT implicated genetic variants in fibrinogen alpha (FGA) and FGG genes as genetic determinants for VT through the reduction of circulating plasma fibrinogen γ′ levels.5 In this study, homozygous carriership of an FGG risk haplotype (FGG-H2) was associated with an increased risk for VT (odds ratio = 2.4; 95% confidence interval, 1.5-3.9) and, furthermore, reduced fibrinogen γ′ levels and reduced ratios of fibrinogen γ′ to total fibrinogen. This study was further corroborated by a recent study by Grünbacher et al,6 which showed an increased risk for carriers of the FGG 10034 TT genotype and VT (odds ratio = 2.01; 95% confidence interval, 1.23-3.31, P = .006) in 358 patients and 783 controls, further supporting the importance of genetic variants in FGG in the pathogenesis of VT. However, similar studies in VT of pediatric onset are lacking. VT in children is a rare event that probably occurs in neonates and children with underlying conditions, such as cancer, sepsis, autoimmune disease, and congenital heart disease, or as a consequence of invasive therapeutic procedures. Prominent complicating features of VT in children include lack of thrombus resolution and development of postthrombotic syndrome.7,–9 Because of the low incidence of pediatric VT in the general population (0.07-0.14/10 000 population children; ∼ 5/10 000 hospital admissions of children), studies addressing the impact of genetic modifiers of thrombosis on the incidence of pediatric VT are limited by the relatively small number of patients and therefore often remain inconclusive. Meta-analyses of available data suggest that inherited thrombophilic mutations in coagulation factors identified in adult patients (ie, factor VLeiden [FVLeiden] G1691A, prothrombin G20210A, deficiency of protein S, protein C, and antithrombin) are also the most common genetic determinants of pediatric first VT and ischemic stroke.7,9,10 The contribution of fibrinogen gene variation to the incidence of VT in children, and the association with the relative abundance of fibrinogen γ chain isoforms in plasma is unknown. The goal of the current study was to examine the association between genetic variation in FGG and FGA genes, plasma levels of γA/γ′ fibrinogen, and thromboembolic disease (VT and nonvascular thromboembolic stroke [TS]) in children.
Methods
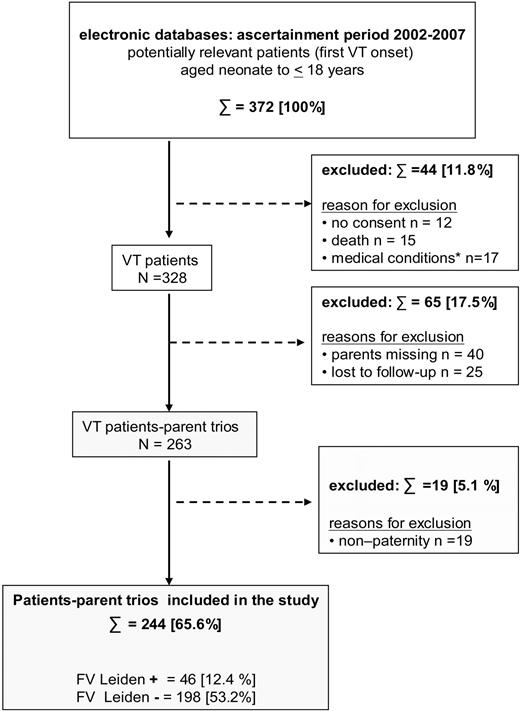
In the German study center Muenster, plasma and DNA samples of 244 families of children with VT (Figure 1) and 268 families of children with nonvascular TS (replication cohort),11 that is, samples of the propositus, nonaffected brothers or sisters, and biologic parents, were collected between January 2002 and December 2007. The present study was performed in accordance with the ethical standards laid down in the updated version of the 1964 Declaration of Helsinki and was approved by the medical ethics committee of the University of Münster, Germany. With written parental consent, term neonates and children with confirmed diagnosis of VT not older than 18 years at onset, biologic brothers and sisters, and available parents were enrolled. Premature birth (≤ 36 gestational weeks), patients older than 18 years at onset, and children with a first ischemic stroke of vascular origin were excluded. In addition, children with missing parents, families with nonpaternity, children lost to follow-up, and patients without parental consent were excluded. Additional exclusion criteria were ongoing liver, renal, or inflammatory diseases, malignancies, and concurrent treatment regimens known to influence fibrinogen levels. Inclusion and exclusion criteria are shown in Figure 1. The replication study sample with pediatric TS and criteria for group assignment to vascular stroke were described in detail elsewhere.11,12
Flow chart of patient-parent trio selection. Inclusion and exclusion criteria for patients enrolled in the study are shown.
Flow chart of patient-parent trio selection. Inclusion and exclusion criteria for patients enrolled in the study are shown.
Blood sample collection
Blood sample collection from patients was done in the morning after a 12-hour fasting period (infants fasted 4-6 hours); samples were drawn by peripheral venipuncture into plastic tubes containing one-tenth by volume of 3.8% trisodium citrate (Sarstedt) and were immediately placed on melting ice. The blood samples from patients were collected 6 to 12 months after the acute thrombotic event and at least 6 weeks apart from anticoagulation. Platelet-poor plasma was prepared by centrifugation at 3000g and 4°C for 2 × 20 minutes, aliquoted in polystyrene tubes, stored at −70°C, and thawed immediately before assay. DNA extraction was performed by a spin column procedure (QIAGEN) according to the manufacturer's instructions.
Fibrinogen and fibrinogen gamma γ′ antigen measurements
Total fibrinogen was determined according to the Clauss method using Dade thrombin reagent (Dade-Behring). The test was performed on a Dade Behring BCS analyzer. Fibrinogen γ′ antigen levels were measured by enzyme-linked immunosorbent assay as described previously13 with the following modifications: Fibrinogen gamma′ chains were detected with the L2B antibody that recognizes both the full-length as well as the truncated form of the γ′ chain variant. Standards were prepared from admixtures of purified peak I and II preparations of fibrinogen corresponding to a range between 2% and 20% gamma′ content, and all patient samples fell into this concentration range. Horseradish peroxidase-labeled L2B-antibody binding to immobilized patient fibrinogen was calculated from the Vmax of color development over 15 minutes.
Genetic analysis
We identified 7 haplotype tagging single nucleotide polymorphisms (htSNPs) capturing 97.5% of the genetic variation in FGG and FGA genes using the genotype information from Centre d'Etude du Polymorphisme Humain families available from HAPMAP (www.hapmap.org) and the SNPtagger-tool as implemented in HAPLOVIEW.14 These htSNPs are composed of 3 SNPs located in FGG (rs2066860, rs2066861, rs1049636) and 4 SNPs in FGA (rs2070011, rs2070014, rs2070016, rs6050; Thr312Ala), and are in accordance with the htSNPs recently reported by Kardys et al.4 Genotyping was performed using the TaqMan allelic discrimination method on a 384-well H7900 (Applied Biosystems) using 2 ng of genomic DNA. For quality control, each plate contained 8 positive Centre d'Etude du Polymorphisme Humain controls and 8 no-template controls to ensure genotyping accuracy. Genotyping efficiency was more than 99.5%. In addition, the presence or absence of the FVLeiden mutation was determined using standard laboratory techniques.15,16
Statistical analysis
Hardy-Weinberg equilibrium for each htSNP was tested using χ2 analysis across all samples. Family-based association was determined using the transmission disequilibrium test (TDT)17 in 244 trios comprising unaffected parents and the affected child; haplotypes were inferred using the Expectation Maximization Algorithm as implemented in Haploview (Version 4.0).14 Significance of association was assessed using a Pearson χ2 test and corrected for multiple testing using 10 000 permutations as implemented in Haploview. TDT analyses were repeated after stratification for presence of at least one copy of the FVLeiden mutation in the affected child (46 families positive for the FVLeiden mutation, 198 families without FVLeiden). For correlation analyses between associated FGG haplotypes and fibrinogen γ′ to total fibrinogen levels in the affected children, the individual haplotypes were reconstructed based on phase because genotype information was available for both parents and the affected child. Distribution of total fibrinogen and fibrinogen γ′ levels were assessed using the Kolmogorov-Smirnov test for normalcy to ensure a normal distribution as a prerequisite for parametric analyses. Correlation analyses were performed using one-way analysis of variance, followed by the appropriate post hoc test (Student t test). P values were adjusted for multiple testing using the Bonferroni method (haplotypes, FVLeiden carriership [yes/no], 2 phenotypes = 8 comparisons).
Results
Association between fibrinogen haplotypes and pediatric VT
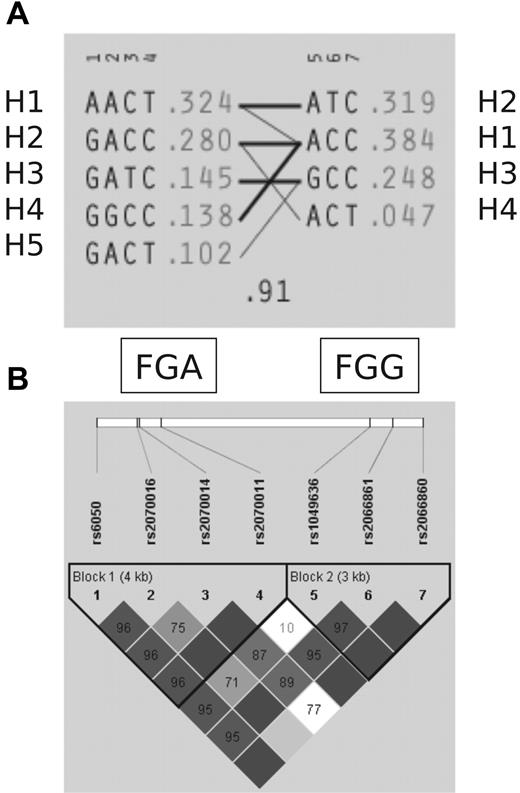
Genotyping of 244 families for VT (786 persons) showed that the selected 7 htSNPs identified the 4 most common haplotypes in FGG and the 5 most common haplotypes in FGA, and thus captures 97.5% of the genetic variation present in haplotypes at a frequency more than 1% (Figure 2A). For all SNPs, the distribution of the genotypes was in Hardy-Weinberg equilibrium and similar to those reported by previous studies.4,5 Consistent with previous observations,4,5 a high degree of linkage disequilibrium was detected between FGA and FGG (Figure 2B). However, in our study, recombination events were detected between rs2070011 and rs1049636 (D′ = 0.1) that may affect haplotype structure of the fibrinogen gene cluster (Figure 2A).
LD structure and haplotypes in FGA and FGG genes. (A) Five FGA (H1-H5) and 4 FGG (H1-H4) haplotypes are defined by 7 htSNPs capturing 97.5% of the genetic variation in the extended linkage disequilibrium (LD) block harboring FGA and FGG. (B) LD structure underlying FGA and FGG.
LD structure and haplotypes in FGA and FGG genes. (A) Five FGA (H1-H5) and 4 FGG (H1-H4) haplotypes are defined by 7 htSNPs capturing 97.5% of the genetic variation in the extended linkage disequilibrium (LD) block harboring FGA and FGG. (B) LD structure underlying FGA and FGG.
Single-point TDT identified 3 significantly associated SNPs with VT, rs6050 [Thr312Ala] (ratio of transmissions to nontransmissions of the overtransmitted allele [T:U] 84:51, χ2 = 8.067, P = .004), rs2070011 (T:U 102:64, χ2 = 8.699, P = .003) in FGA, and rs2066861 (T:U 80:52, χ2 = 5.939, P = .015) in FGG (Table 1). For each SNP, a significant overtransmission to affected offspring was observed, implying that these SNPs confer a risk for juvenile VT. Subsequent haplotype association analysis revealed that haplotype H1 of the FGA gene (AACT) and haplotypes H2 (ATC) were overtransmitted to VT patients, whereas H3 (GCC) of the FGG gene was significantly undertransmitted to VT patients (FGA: H1, T:U 60:32.6, χ2 = 9.134, P = .05; FGG: H2, T:U 85:47, χ2 = 10.939, P = .032; H3, T:U 28:48, χ2 = 5.277, P = .022; Table 2). The associations for H1 in FGA and H3 in FGG remained significant after permutation testing using 10 000 permutations (FGA: H1, χ2 = 9.137, P = .024; FGG: H3, χ2 = 8.523, P = .037). These data document that the FGA-H1 and the FGG-H2 haplotypes are overrepresented in pediatric patients with VT. Conversely, the FGG-H3 haplotype was significantly undertransmitted to affected offspring in our pediatric VT cohort and might thus represent a protective haplotype.
Single-point association (TDT) between variants in FGA and FGG and juvenile VT
| SNP ID no. . | MAF . | Overtransmitted . | T:U . | χ2 . | P . |
|---|---|---|---|---|---|
| All VT | |||||
| rs6050 | 0.319 | A | 84:51 | 8.067 | .004* |
| rs2070016 | 0.148 | G | 42:41 | 0.012 | .913 |
| rs2070014 | 0.154 | C | 46:31 | 2.922 | .087 |
| rs2070011 | 0.419 | T | 102:64 | 8.699 | .003* |
| rs1049636 | 0.252 | A | 62:52 | 0.877 | .349 |
| rs2066861 | 0.317 | T | 80:52 | 5.939 | .015* |
| rs2066860 | 0.048 | C | 15:10 | 1 | .317 |
| FVLeiden-negative families | |||||
| rs6050 | 0.315 | A | 68:38 | 8.491 | .004* |
| rs2070016 | 0.142 | A | 33:30 | 0.143 | .705 |
| rs2070014 | 0.162 | C | 38:26 | 2.25 | .134 |
| rs2070011 | 0.41 | T | 85:47 | 10.939 | .001* |
| rs1049636 | 0.257 | A | 50:47 | 0.093 | .761 |
| rs2066861 | 0.321 | T | 68:40 | 7.259 | .007* |
| rs2066860 | 0.048 | C | 12:08 | 0.8 | .371 |
| FVLeiden-positive families | |||||
| rs6050 | 0.325 | A | 16:11 | 0.926 | .336 |
| rs2070016 | 0.171 | G | 11:08 | 0.474 | .491 |
| rs2070014 | 0.128 | C | 08:04 | 1.333 | .248 |
| rs2070011 | 0.442 | T | 17:15 | 0.125 | .724 |
| rs1049636 | 0.237 | A | 12:04 | 4 | .045 |
| rs2066861 | 0.295 | T | 12:10 | 0.182 | .670 |
| rs2066860 | 0.045 | C | 03:02 | 0.2 | .655 |
| SNP ID no. . | MAF . | Overtransmitted . | T:U . | χ2 . | P . |
|---|---|---|---|---|---|
| All VT | |||||
| rs6050 | 0.319 | A | 84:51 | 8.067 | .004* |
| rs2070016 | 0.148 | G | 42:41 | 0.012 | .913 |
| rs2070014 | 0.154 | C | 46:31 | 2.922 | .087 |
| rs2070011 | 0.419 | T | 102:64 | 8.699 | .003* |
| rs1049636 | 0.252 | A | 62:52 | 0.877 | .349 |
| rs2066861 | 0.317 | T | 80:52 | 5.939 | .015* |
| rs2066860 | 0.048 | C | 15:10 | 1 | .317 |
| FVLeiden-negative families | |||||
| rs6050 | 0.315 | A | 68:38 | 8.491 | .004* |
| rs2070016 | 0.142 | A | 33:30 | 0.143 | .705 |
| rs2070014 | 0.162 | C | 38:26 | 2.25 | .134 |
| rs2070011 | 0.41 | T | 85:47 | 10.939 | .001* |
| rs1049636 | 0.257 | A | 50:47 | 0.093 | .761 |
| rs2066861 | 0.321 | T | 68:40 | 7.259 | .007* |
| rs2066860 | 0.048 | C | 12:08 | 0.8 | .371 |
| FVLeiden-positive families | |||||
| rs6050 | 0.325 | A | 16:11 | 0.926 | .336 |
| rs2070016 | 0.171 | G | 11:08 | 0.474 | .491 |
| rs2070014 | 0.128 | C | 08:04 | 1.333 | .248 |
| rs2070011 | 0.442 | T | 17:15 | 0.125 | .724 |
| rs1049636 | 0.237 | A | 12:04 | 4 | .045 |
| rs2066861 | 0.295 | T | 12:10 | 0.182 | .670 |
| rs2066860 | 0.045 | C | 03:02 | 0.2 | .655 |
MAF indicates minor allele frequency; and T:U, ratio of transmissions to nontransmissions.
P value indicates statistical significance.
Association between FGA and FGG haplotypes and juvenile VT
| Block . | Haplotype* . | Frequency . | T:U* . | χ2 . | P . |
|---|---|---|---|---|---|
| All VT | |||||
| FGA haplotypes | |||||
| H1 | AACT | 0.324 | 62.0: 32.6 | 9.134 | .002‡ |
| H2 | GACC | 0.28 | 39.7: 51.7 | 1.576 | .209 |
| H3 | GATC | 0.145 | 17.0: 32.0 | 4.591 | .0321 |
| H4 | GGCC | 0.138 | 27.3: 23.3 | 0.317 | .574 |
| H5 | GACT | 0.102 | 19.0: 22.4 | 0.278 | .598 |
| FGG haplotypes | |||||
| H1 | ACC | 0.384 | 51.0: 58.0 | 0.447 | .508 |
| H2 | ATC | 0.319 | 60.0: 32.0 | 8.523 | .003‡ |
| H3 | GCC | 0.248 | 28.0: 48.0 | 5.277 | .022 |
| H4 | ACT | 0.047 | 9.0: 9.0 | 0 | 1 |
| FVLeiden-negative families | |||||
| FGA haplotypes | |||||
| H1 | AACT | 0.308 | 55.0: 24.3 | 11.85 | .001‡ |
| H2 | GACC | 0.281 | 29.0: 52.0 | 6.532 | .011 |
| H3 | GATC | 0.154 | 17.0: 22.0 | 0.64 | .423 |
| H4 | GGCC | 0.139 | 20.0: 20.0 | 0 | 1 |
| H5 | GACT | 0.105 | 17.0: 16.7 | 0.003 | .953 |
| FGG haplotypes | |||||
| H1 | ACC | 0.378 | 37.1: 57.0 | 4.233 | .040 |
| H2 | ATC† | 0.315 | 54.9: 24.0 | 12.157 | .001‡ |
| H3 | GCC | 0.256 | 30.9: 36.0 | 0.38 | .537 |
| H4 | ACT | 0.049 | 6.0: 11.0 | 1.471 | .225 |
| Block . | Haplotype* . | Frequency . | T:U* . | χ2 . | P . |
|---|---|---|---|---|---|
| All VT | |||||
| FGA haplotypes | |||||
| H1 | AACT | 0.324 | 62.0: 32.6 | 9.134 | .002‡ |
| H2 | GACC | 0.28 | 39.7: 51.7 | 1.576 | .209 |
| H3 | GATC | 0.145 | 17.0: 32.0 | 4.591 | .0321 |
| H4 | GGCC | 0.138 | 27.3: 23.3 | 0.317 | .574 |
| H5 | GACT | 0.102 | 19.0: 22.4 | 0.278 | .598 |
| FGG haplotypes | |||||
| H1 | ACC | 0.384 | 51.0: 58.0 | 0.447 | .508 |
| H2 | ATC | 0.319 | 60.0: 32.0 | 8.523 | .003‡ |
| H3 | GCC | 0.248 | 28.0: 48.0 | 5.277 | .022 |
| H4 | ACT | 0.047 | 9.0: 9.0 | 0 | 1 |
| FVLeiden-negative families | |||||
| FGA haplotypes | |||||
| H1 | AACT | 0.308 | 55.0: 24.3 | 11.85 | .001‡ |
| H2 | GACC | 0.281 | 29.0: 52.0 | 6.532 | .011 |
| H3 | GATC | 0.154 | 17.0: 22.0 | 0.64 | .423 |
| H4 | GGCC | 0.139 | 20.0: 20.0 | 0 | 1 |
| H5 | GACT | 0.105 | 17.0: 16.7 | 0.003 | .953 |
| FGG haplotypes | |||||
| H1 | ACC | 0.378 | 37.1: 57.0 | 4.233 | .040 |
| H2 | ATC† | 0.315 | 54.9: 24.0 | 12.157 | .001‡ |
| H3 | GCC | 0.256 | 30.9: 36.0 | 0.38 | .537 |
| H4 | ACT | 0.049 | 6.0: 11.0 | 1.471 | .225 |
T:U indicates ratio of transmissions to nontransmissions.
The haplotype association for FVLeiden-positive families is not available because of the small sample size.
The H2-ATC haplotype in FGG is equivalent to H2-GAT haplotype described by Uitte de Willige et al.5 The different annotation is the result of TaqMan primers being designed on the opposite DNA strand.
P value indicates statistical significance.
The strong linkage disequilibrium detected between the FGA-H1 and FGG-H2 risk haplotypes (Figure 2B) raised the possibility that these haplotypes are largely coinherited. Phase-adjusted haplotype analysis showed indeed that a recombination event between FGA-H1 and FGG-H2 had occurred in less than 10% of children.
Replication of association in thromboembolic pediatric stroke
To determine the association of FGA and FGG haplotypes with thromboembolic nonvascular stroke (TS), a corresponding analysis was performed in an independent family-based cohort of 268 nuclear families with TS. In FGA, rs6050 [Thr312Ala] was significantly associated with TS (T:U 95:69, χ2 = 4.122, P = .042). In FGG, rs2066861 showed a nonsignificant trend toward association (T:U 96:73, χ2 = 3.13, P = .076), and rs2066860 was significantly associated with TS (T:U 16:05, χ2 = 5.762, P = .016). For all 3 SNPs, the same allele was overtransmitted to affected offspring as observed in VT. The subsequent haplotype association analysis revealed that haplotype H1 of the FGA gene (AACT) and haplotypes H2 (ATC) and H4 (ACT) of the FGG gene were significantly associated with TS (FGA: H1, T:U 100.7:66.7, χ2 = 6.916, P = .008; FGG: H2, T:U 97:72.4, χ2 = 3.57, P = .05; H4, T:U 5:19, χ2 = 5.671, P = .018), replicating the haplotype association of FGA-H1 and FGG-H2 risk haplotypes, but not the putative protective H3 haplotype (T:U 88.4:88.3, χ2 = 0.00, P = .993).
To increase the statistical power of the study, we examined whether combining the VT and TS study populations would strengthen or weaken the association of this haplotype with thromboembolic disease. Given the allele frequency of 0.41 for the risk allele (eg, rs6050 or rs2066861) and a genotype relative risk of 1.5 and 2.0 for heterozygous and homozygous risk allele carriers, respectively, combining all 512 families from both study samples is predicted to enhance the statistical probability to detect association at an α = 0.05 through TDT analysis to 79.8%. Single-point TDT analysis of the combined pediatric VT and TS study sample documented significant association of genetic variants in FGA and FGG at a level of confidence comparable with (rs2070011, P = .004) or exceeding (rs6050, P = .001; rs2066861, P = .003; rs2066860, P = .018) the level measured in the VT or TS population alone. Importantly, in the corresponding haplotype analysis, the strength of association of the putative risk haplotypes FGA-H1 and FGG-H2 with TS was augmented on pooling the TS and VT population (FGA-H1: P = .001; FGG-H2: P = .002).
This analysis indicates that the VT-risk haplotypes FGA-H1 and FGG-H2 also modify the incidence of TS. Conversely, association analysis of FGG-H3 and FGG-H4 produces divergent outcomes in VT (H3 protective/undertransmitted; H4 neutral) and TS patients (H3 neutral; H4 protective/undertransmitted).
Genetic interaction between FVLeiden and FGA and FGG variants
Consistent with existing data,8 the FVLeiden mutation is significantly associated with VT in our family cohort (T:U 26:13, χ2 = 4.33, P = .037). To detect potentially confounding interactions between genetic variations in FGG and FGA and the FV gene, we investigated whether the observed FGA and FGG haplotype associations with VT were affected by FVLeiden carriership. Therefore, we stratified our cohort on FVLeiden carriership, identifying 46 FVLeiden-positive trios and 198 FVLeiden-negative trios, and repeated the TDT analysis. The association between FGA and FGG and VT was more pronounced in FVLeiden-negative trios (FGA: H1, T:U 55:24, P = .001; FGG: H2, T:U 55:24, P = .001) but was not significant in FVLeiden-positive trios (Tables 1, 2). In addition, the association of FGG-H3 with VT was lost in FVLeiden-negative carriers after stratification.
Because the relatively small number (n = 46) of FVLeiden-positive families may provide insufficient power to detect association, we repeated the analysis in the combined study samples of VT and TS, comprising 420 FVLeiden-negative and 92 FVLeiden-positive families. TDT analysis of the combined study sample (512 families) resulted in a significant association of genetic variants in FGA and FGG in FVLeiden-negative trios, in both single point (FGA: rs6050, T:U 148:99, P = .002; rs2070011, T:U 176:118, P = .001; FGG: rs2066861, T:U 145:105, P = .011; rs2066860, T:U 22:11, P = .05) and haplotype analysis (FGA: H1, P = .001; FGG: H2, P = .007). Single-point TDT in FVLeiden-positive trios showed a trend for association for rs6050 [Thr312Ala] in FGA (T:U 31:19, P = .089) and rs2066861 in FGG (T:U 31:18, P = .063), and a significant association for rs1049636 (T:U 32:12, P = .003). Pooling VT and TS families stratified for FVLeiden status thus strengthened the association of the risk haplotypes FGA-H1 and FGG-H2 in FVLeiden-negative trios but failed to compensate for the loss of association in FVLeiden carriers.
Fibrinogen haplotypes and total fibrinogen and fibrinogen γ′ levels in VT children
Genetic variation in the FGG gene and associated disease risk has been suggested to correlate with plasma concentrations of total fibrinogen, as well as altered absolute and proportional abundance of the fibrinogen γ′ isoform in adult patients with VT or ischemic stroke.2,5,18 To examine how carriership for FGG risk haplotypes correlated with total fibrinogen and γ′ levels in the pediatric VT study population, fibrinogen γ′ levels and total fibrinogen levels were measured in 207 VT children. Total plasma fibrinogen levels were distributed normally, as judged from Kolmogorov-Smirnov testing. Because genotype information for both parents and the affected child was known, individual haplotypes could be assigned to 98% of all children (n = 202). This enabled parametric statistical analysis to investigate the correlation between haplotype status on one hand, and fibrinogen, fibrinogen γ′, and the percentage of fibrinogen γ′ in relation to total fibrinogen, on the other hand.
Total plasma fibrinogen levels were identical in non-H2 carriers (n = 92) and carriers of at least one copy of the FGG-H2 risk haplotype (n = 110; 291.1 ± 87.6 vs 283.0 ± 92.3, P = .26). However, fibrinogen γ′ levels and γ′ content within total fibrinogen (% γ′) were significantly lower in H2 carriers (γ′ levels: 22.7 ± 13.7 vs 26.8 ± 12.0, P = .013; % γ′: 7.63 ± 3.05 vs 9.46 ± 3.17, P = 2.3 × 10−5, Bonferroni-corrected P = .001). This observation was independent of FVLeiden carriership (data not shown). Carriers of at least one copy of the H3 haplotype (n = 82) compared with noncarriers (n = 120) exhibited significantly lower total plasma fibrinogen levels (270.4 ± 109.0 vs 299.1 ± 100.03, P = .012ç Bonferroni-corrected P = not significant), but increased γ′ content within total fibrinogen (% γ′: 9.21 ± 3.09 vs 7.95 ± 3.23, P = .003, Bonferroni-corrected P = .024). These effects were also detected in FVLeiden-negative H3 carriers (fibrinogen: 265.7 ± 69.2 vs 298.1 ± 94.5, P = .009, % γ′: 9.07 ± 3.11 vs 7.79 ± 3.01, P = .005, Bonferroni-corrected P = .038) but did not reach significance in FVLeiden-positive H3 carriers (fibrinogen: 292.6 ± 69.3 vs 302.4 ± 118.9, P = .388; % γ′ content (%): 9.92 ± 3.03 vs 8.52 ± 3.89, P = .125).
Homozygous carriership of the FGG-H2 risk haplotype was associated with the lowest fibrinogen γ′ content, in both absolute and relative terms (Table 3). In compound heterozygous carriers of one H2 allele and any of the other 3 FGG haplotypes, absolute and relative γ′ levels were on average higher than in H2/H2 homozygotes but lower than in non-H2 carriers (Table 3). Homozygous carriers of the (undertransmitted) FGG H3 haplotype show the highest relative fibrinogen γ′ content, in part because of overall lower total fibrinogen levels. A similar positive correlation between FGG-H3 carrier status and relative abundance of γ′ fibrinogen (P = .001, Bonferroni-corrected P = .001) is seen in compound heterozygotes carrying one H3 allele and one H2 allele. Congruent with studies by others,5,19 carriership for any risk haplotype was associated weakly, if at all, with total plasma fibrinogen. These outcomes are consistent with the notion that the VT and TS risk allele FGG-H2 is associated with a lower γ′/total fibrinogen ratio, independent of absolute fibrinogen levels.
Correlation of FGG haplotypes with fibrinogen and fibrinogen γ′ content in juvenile VT subjects
| Haplotype . | n . | Fibrinogen . | γ′ content, % . | γ′ absolute . |
|---|---|---|---|---|
| H2/H2 | 26 | 285.1 ± 70.7 | 6.01 ± 2.25 | 17.30 ± 8.00 |
| H2/H1 | 48 | 297.6 ± 107.5 | 7.67 ± 3.19 | 23.82 ± 17.21 |
| H2/H3 | 30 | 291.6 ± 67.8 | 8.99 ± 2.73 | 26.56 ± 10.80 |
| H2/H4 | 6 | 293.8 ± 84.3 | 7.33 ± 2.83 | 20.56 ± 7.47 |
| H1/H1 | 29 | 316.1 ± 125.9 | 9.78 ± 2.95 | 30.76 ± 13.66 |
| H1/H3 | 33 | 253 ± 67.27 | 9.01 ± 3.89 | 23.08 ± 19.30 |
| H1/H4 | 11 | 296.0 ± 56.2 | 9.33 ± 3.60 | 27.21 ± 9.90 |
| H3/H3 | 19 | 275.6 ± 71.3 | 9.84 ± 3.15 | 27.19 ± 10.71 |
| H3/H4 | 3 | 253 ± 23.1 | 8.37 ± 4.13 | 20.61 ± 8.83 |
| FVLeiden-negative | ||||
| FGG-H2 | 83 | 282 ± 72 | 7.5 ± 3 | 21.4 ± 11 |
| Other | 72 | 279 ± 96 | 9.2 ± 3.1 | 25.9 ± 12 |
| H2 vs other, P | NS | .001 | .02 | |
| FVLeiden carrier | ||||
| FGG-H2 | 21 | 307 ± 125 | 7.5 ± 4 | 23.4 ± 19 |
| Other | 18 | 300 ± 75 | 11 ± 3 | 32.8 ± 11 |
| H2 vs other, P | NS | .002 | .06 |
| Haplotype . | n . | Fibrinogen . | γ′ content, % . | γ′ absolute . |
|---|---|---|---|---|
| H2/H2 | 26 | 285.1 ± 70.7 | 6.01 ± 2.25 | 17.30 ± 8.00 |
| H2/H1 | 48 | 297.6 ± 107.5 | 7.67 ± 3.19 | 23.82 ± 17.21 |
| H2/H3 | 30 | 291.6 ± 67.8 | 8.99 ± 2.73 | 26.56 ± 10.80 |
| H2/H4 | 6 | 293.8 ± 84.3 | 7.33 ± 2.83 | 20.56 ± 7.47 |
| H1/H1 | 29 | 316.1 ± 125.9 | 9.78 ± 2.95 | 30.76 ± 13.66 |
| H1/H3 | 33 | 253 ± 67.27 | 9.01 ± 3.89 | 23.08 ± 19.30 |
| H1/H4 | 11 | 296.0 ± 56.2 | 9.33 ± 3.60 | 27.21 ± 9.90 |
| H3/H3 | 19 | 275.6 ± 71.3 | 9.84 ± 3.15 | 27.19 ± 10.71 |
| H3/H4 | 3 | 253 ± 23.1 | 8.37 ± 4.13 | 20.61 ± 8.83 |
| FVLeiden-negative | ||||
| FGG-H2 | 83 | 282 ± 72 | 7.5 ± 3 | 21.4 ± 11 |
| Other | 72 | 279 ± 96 | 9.2 ± 3.1 | 25.9 ± 12 |
| H2 vs other, P | NS | .001 | .02 | |
| FVLeiden carrier | ||||
| FGG-H2 | 21 | 307 ± 125 | 7.5 ± 4 | 23.4 ± 19 |
| Other | 18 | 300 ± 75 | 11 ± 3 | 32.8 ± 11 |
| H2 vs other, P | NS | .002 | .06 |
NS indicates not significant.
Discussion
Results of the current genetic study of 512 families with at least one child affected by childhood-onset VT or TS indicate that carriership for the FGG H2 haplotype is significantly associated with pediatric VT as well as TS. Measurements of total fibrinogen and the γ′ isoform of FGG suggest a gene dose effect of FGG alleles on the γ′:fibrinogen ratio, independent of absolute plasma fibrinogen levels, with highest relative γ′ levels in homozygous FGG-H3 carriers, and lowest relative γ′ levels in children homozygous for the FGG-H2 risk allele. These data are congruent with earlier findings characterizing the FGG H2 allele and associated diminished relative levels of γ′ fibrinogen as a risk factor for VT in adults.5,19 Although a recent case-control study of adults failed to detect an association of the FGG H2 risk haplotype with VT, this lack of association might be ascribed to the relatively small sample size (124 cases and 125 controls) and therefore most probably constitutes a type II error (missed association). The current analysis further detected a statistically significant negative correlation of FGG-H3 carriership with VT. This finding dovetails evidence from one previous study20 that the FGG H3 haplotype may constitute a protective factor for ischemic stroke. We note that, in the original study by Uitte de Willige et al,5 the FGG H3 haplotype was also observed more frequently in controls than in VT patients and was associated with a decreased VT risk (odds ratio = 0.8; 95% confidence interval, 0.6-1.0).
In aggregate, these findings suggest that (1) the plasma γ′/fibrinogen ratio moderately alters the population risk of VT and ischemic stroke and (2) variations in the plasma γ′/fibrinogen ratio are in part determined by genetic variations in the FGG gene, with highest and lowest γ′ ratios associated in a gene dose-dependent manner with the FGG H3 and H2 alleles, respectively.
Our present findings further show that the most frequent FGA-H1 haplotype is overrepresented in pediatric VT patients and in children with nonvascular stroke. In adults, the Ala312Thr polymorphism in FGA has been linked to VT19,21,22 and poststroke mortality in patients with atrial fibrillation.23 The FGA H1 haplotype is uniquely detected by the presence of the A-allele of the SNP rs6050, which encodes the Ala312 variant of the fibrinogen Aα chain, whereas the other 4 major FGA haplotypes are composed of the G-allele of rs6050, encoding the Thr312 Aα chain. Analysis of clots formed from purified fibrinogen Ala312 and Thr312 showed that Ala312 fibrin fibers are thicker, produce clots with reduced elasticity, and exhibit a higher degree of cross-linking mediated by factor XIII.24
The high degree of linkage disequilibrium between FGG and FGA gene haplotypes confounds the assignment of disease risk to specific alleles and, in general, complicates establishing the association between genetic variations in the fibrinogen gene locus, level, and composition of fibrinogen. Specifically, studies examining the effect of putative FGG H2 risk haplotype associated with diminished γ′ chain production relative to total fibrinogen recognized that this haplotype is in a significant portion of persons coinherited with a second polymorphism located in the FGA gene (FGA Thr312Ala; rs6050). We show that the same is true in our pediatric study population, where the FGA H1 variant is coinherited with the FGG H2 risk haplotype in 188 of 200 children. These observations highlight that FGA-H1 (Ala312) and FGG-H2 (low γ′/γ ratio) are in the majority of persons coinherited. As briefly discussed in 2 previous studies,5,20 this implies, first, that population-based calculations of the risk conferred by these haplotypes must consider haplotype combinations, rather than a model of independent risk factors. Indeed, comparison of patients carrying the combined FGA-H1/FGG-H2 haplotypes (n = 104) versus non-FGA-H1/FGG-H2 carriers (n = 90) yields identical results as obtained from an analysis of the individual haplotype associations with total fibrinogen (287 ± 85 mg/dL vs 283 ± 92, P = not significant), absolute levels of γ′ (21.8 ± 12 vs 27.2 ± 12 mg/dL; P = .002), and γ′/fibrinogen ratio (7.5% ± 3% vs 9.6% ± 3.1%; P = .001).
Recent experiments in mice genetically altered to produce a human γ′ fibrin variant capable of high-affinity interaction with thrombin provided direct experimental evidence that γ′/γA fibrin exerts an anticoagulant effect that ameliorates the prothrombotic effect of the FVLeiden mutation.25 Stratification of families according to FVLeiden carriership showed that the genetic risk attributable to FGA and FGG variants is influenced by the presence or absence of the FVLeiden-mutation: the association of FGG-H2 with a lower γ′/fibrinogen ratio, and with borderline significance (P = .057) also of absolute γ′ levels, was clearly detectable in both FVLeiden-negative and -positive persons and was again independent of total fibrinogen levels (Table 3). The loss of genetic association with disease might be the result of a lack of statistical power to detect a weak association of FGA/FGG haplotypes in the presence of a comparably strong risk factor, such as the FVLeiden mutation. However, a different SNP (rs1039636; data not shown) was associated with VT and/or TS exclusively in FVLeiden carriers, but not in FVLeiden-negative families, indicating that our study was sufficiently powered to detect such positive interactions.
Although the design of the current study inherently does not enable calculations of odds ratios, estimates of the genotype relative risk are consistent with results from population-based association studies that estimate the risk conferred by FGA-H1/FGG-H2 carriership in the range of 1.1 to 1.8. The FGA-H1/FGG-H2 risk alleles therefore only modestly affect overall disease incidence and are in all likelihood not informative for predicting thrombosis risk in a given person. However, genotyping for carriership of the FGA-H1/FGG-H2 alleles could in theory identify a subpopulation of patients whose blood clots exhibit properties distinct from those formed in noncarriers of these alleles. The overall biochemical properties postulated for Ala312 fibrin produced in FGA H1 carriers are remarkably similar to the biochemical effects predicted for diminished levels of γ′ fibrinogen on clot structure, including partial resistance to fibrinolysis,26 enhanced mechanical clot stiffness, and increased fiber diameter. In addition, thrombin bound to the γ′ isoform is relatively resistant to anticoagulant treatment with heparin.27,28 Thus, in vivo formed clots in FGA-H1/FGG-H2 persons might combine, possibly in an exaggerated manner, the properties of clots documented ex vivo for γ′ fibrin or Ala312 fibrin. Fibrin γ′ chains not only interact with thrombin but also contain a nearby binding site for fXIII zymogen. The interaction of fibrin-associated fXIII and thrombin may contribute to the altered clot characteristics of fibrin with a high content of γ′ chains by altering the kinetics of fXIII activation by thrombin.29,30 We note in this context that the fXIII Leu34 variant, which has been identified as a protective trait in thrombotic disorders, produces similar alterations in fibrin structure as high levels of γ′ fibrin.29 In vitro experiments with purified fibrinogen suggest that an increased rate of thrombin generation, as probably occurs in FVLeiden carriers, may override the biochemical differences between the structures of Ala312 and Thr312 fibrin, or γA/γA and γ′/γA fibrin,31,,–34 providing a theoretical explanation how the FVLeiden mutation might mitigate the risk conferred by FGA-H1/FGG-H2.
Taken together, the effects of polymorphisms in fXIII, fibrinogen A, and fibrinogen γ chain genes on thrombosis may converge on a common biochemical pathway that defines fibrin structure and susceptibility of formed fibrin to fibrinolysis. The broader significance of these findings for identifying patients at risk and the therapeutic approach to thrombosis remain to be investigated.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: T. Seehafer, S.T., and T. Schulte conducted the genotyping and data management; H.W. and I.H. performed the biochemical studies on fibrinogen gamma′ variants; and M.S. and U.N.-G. were responsible for the statistical analyses. All authors had full access to the data and took part in the design, execution, and data analysis, and in writing the report.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Monika Stoll, Genetic Epidemiology of Vascular Disorders, Leibniz-Institute for Arteriosclerosis Research, Domagkstrasse 3, 48149 Muenster, Germany; e-mail: mstoll@uni-muenster.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal