The glycoprotein VI (GPVI)/FcRγ complex is a key receptor for platelet activation by collagen. We describe, for the first time, 2 genetic abnormalities in one patient. This 10-year-old girl presented ecchymoses since infancy, a prolonged bleeding time despite a normal platelet count and no antiplatelet antibodies. Collagen-induced platelet activation was null, whereas GPVI quantification by flow cytometry evidenced an incomplete deficiency. Immunoblotting showed an abnormal migration of residual GPVI, and no FcRγ defect. GPVI DNA sequencing revealed (1) an R38C mutation in exon 3 of one allele and (2) an insertion of 5 nucleotides in exon 4 of the other allele, leading to a premature nonsense codon and absence of the corresponding mRNA. Introduction of the R38C mutation into recombinant GPVI-Fc resulted in abnormal protein migration and a loss of collagen binding. Thus, this composite genetic GPVI deficiency and dysfunction cause absence of platelet responses to collagen and a mild bleeding phenotype.
Introduction
Collagen is a major thrombogenic constituent of the vessel wall. Platelets principally react with collagen via integrin α2β1 and glycoprotein VI (GPVI),1 a member of the immunoglobulin receptor family.2,3 GPVI plays a major role in platelet activation through signaling mediated by the noncovalently associated immunoreceptor tyrosine-based activation motif–containing FcRγ chain.1,4 The extracellular domain of GPVI is composed of 2 Ig-like domains important for collagen binding and receptor dimerization, respectively.5,,–8 GPVI deficiencies9 have been described mostly in patients with immune thrombocytopenic purpura, where autoantibodies are assumed to induce GPVI shedding.10,11 We report the first case of congenital GPVI deficiency with a mild bleeding disorder. This patient, whose platelets failed to respond to collagen, is a compound heterozygote for 2 GPVI mutations: one leads to an incomplete protein deficiency, and the second is a loss of function Arg to Cys substitution in the first GPVI Ig-like loop.
Methods
The supplemental data contain information on methods (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Patients
The patient is a 10-year-old girl who consulted for easy bruising since infancy. Her parents were nonconsanguineous, and there was no family history of bleeding. She had a prolonged bleeding time (Ivy > 15 minutes, PFA-100 closure time on collagen/epinephrine 210 seconds), a normal platelet count (280 G/L), no morphologic abnormalities on blood smears, and no detectable antiplatelet antibodies. von Willebrand factor exploration was in the normal range. All studies were performed with written informed consent according to the Declaration of Helsinki and with approval of the ethical board of Hôpital Necker (Assistance Publique-Hopitaux de Paris).
Platelet function studies
Platelet aggregation was measured on platelet-rich plasma (PRP) and washed platelets.12 Dense granule secretion was quantified using an adenosine triphosphate determination kit. Platelet adhesion to collagen under flow conditions and thrombin generation were measured mainly as described.13,14 GPVI expression was analyzed by flow cytometry and immunoblot using the monoclonal antibody 3J24.2 or 9O12.2 and a human polyclonal antibody.
Genetic studies
Each of the 8 exons and intron-exon junctions of GPVI were amplified by polymerase chain reaction; products were sequenced on both strands. Total mRNA was extracted; cDNA corresponding to exons 3 and 4 was amplified and polymerase chain reaction products sequenced.
R38C mutant
Results and discussion
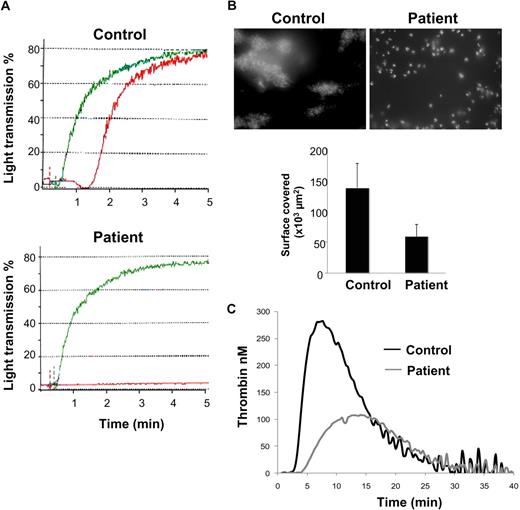
The patient's platelets (PRP and washed platelets) failed to respond to collagen up to 4 μg/mL but aggregated normally in response to adenosine diphosphate, thrombin receptor agonist peptide, arachidonic acid, and ristocetin (Figure 1A, supplemental Figure 1). Activation induced by the GPVI-specific agonist convulxin was also impaired (supplemental Figure 1). Adenosine triphosphate secretion was low in response to 4 μg/mL collagen (404 vs 4727 nM for the patient and control, respectively). The patient's platelet-poor plasma did not inhibit collagen aggregation responses of control platelets or induce spontaneous aggregation.
Platelet functional analysis. (A) Platelet aggregation was measured by turbidimetry on PRP from a control subject and the patient. PRP was stimulated by 5 μM adenosine diphosphate (green) and 1 μg/mL collagen (red). (B) Thrombus formation on collagen under blood flow was analyzed using DiOC6-labeled platelets in whole blood perfused at a shear rate of 500 second−1 over a collagen-coated coverslip during 5 minutes. Thrombus formation was visualized in real-time using an inverted microscope (Leica DMIRB) using a lens at 100× and a 488 nm filter. Images were acquired using a DP30BW camera and CellP software (Olympus) and processed with Histolab software (Microvision). Areas covered by platelet thrombi were measured on 10 different, randomly chosen, microscopic fields. (C) Thrombin generation was triggered by the addition of tissue factor (1 pM) to PRP after incubation with 5 μg/mL collagen. Results are from 1 experiment performed in triplicate, representative of 2 independent experiments.
Platelet functional analysis. (A) Platelet aggregation was measured by turbidimetry on PRP from a control subject and the patient. PRP was stimulated by 5 μM adenosine diphosphate (green) and 1 μg/mL collagen (red). (B) Thrombus formation on collagen under blood flow was analyzed using DiOC6-labeled platelets in whole blood perfused at a shear rate of 500 second−1 over a collagen-coated coverslip during 5 minutes. Thrombus formation was visualized in real-time using an inverted microscope (Leica DMIRB) using a lens at 100× and a 488 nm filter. Images were acquired using a DP30BW camera and CellP software (Olympus) and processed with Histolab software (Microvision). Areas covered by platelet thrombi were measured on 10 different, randomly chosen, microscopic fields. (C) Thrombin generation was triggered by the addition of tissue factor (1 pM) to PRP after incubation with 5 μg/mL collagen. Results are from 1 experiment performed in triplicate, representative of 2 independent experiments.
The capacity of the patient's platelets to form thrombi on collagen in flow conditions was strongly impaired (Figure 1B) with a 43% decreased surface coverage compared with control. A marked defect in collagen-activated platelet-catalyzed thrombin generation was also observed (Figure 1C) with decreased thrombin peak and endogenous thrombin potential. Together, these functional data pointed to a GPVI defect.
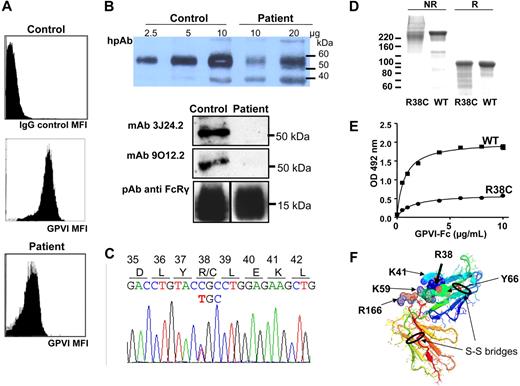
Flow cytometry showed that GPIb and αIIbβ3 and α2β1 integrins were expressed at normal levels on the patient's platelets. In contrast, a severe but incomplete GPVI deficiency was found with 540 copies versus 3076 plus or minus 1332 (mean ± SD from 14 healthy donors) per platelet (Figure 2A). Such a deficiency would not be expected to abolish collagen-induced responses.16 However, by immunoblotting with a human polyclonal antibody, the patient GPVI was detected as a smear (50-60 kDa) and was hardly detectable with monoclonal antibodies 3J24.2 or 9O12.2 (Figure 2B). FcRγ was similar in the patient and control samples. These data suggested that the incomplete deficiency in GPVI was associated with a qualitative defect.
Characterization of the patient's GPVI molecular defect. (A) Flow cytometry on whole blood using the monoclonal antibody 3J24.2 showed decreased GPVI expression on the patient's platelets, compared with control. (B) Serial quantities of proteins in sodium dodecyl sulfate–platelet lysate from the patient or a control were immunoblotted with a human polyclonal anti-GPVI (hpAb). GPVI of the control migrated as a single 58-kDa band, whereas the patient's GPVI migrated as a smear and presented a partial deficiency (note that the 10-μg control GPVI band is saturated; the 35-kDa band is nonspecific). When 10 μg control and patient platelet proteins were immunoblotted with monoclonal antibody 3J24.2 or 9O12.2, the patient's GPVI was hardly detectable. (C) DNA sequence showing the single nucleotide substitution in exon 3 (C172T), resulting in the Arg38 substitution in Cys. (D) Purified recombinant wild-type (WT) and R38C GPVI-Fc were analyzed on sodium dodecyl sulfate–8% acrylamide gels and Coomassie blue staining. WT GPVI-Fc migrated as an approximately 220-kDa band and R38C GPVI-Fc as a smear in nonreducing conditions (NR), and both migrated as a major 95-kDa band after reduction with 5% β-mercaptoethanol (R). (E) Binding of WT and R38C GPVI-Fc to immobilized collagen. Results represent mean ± SD of 2 experiments performed in triplicate. The Kd values are 1.67 ± 0.26 μg/mL and 1.4 ± 0.17 μg/mL for R38C and WT GPVI-Fc, respectively. (F) Three-dimensional structure of the extracellular domain of GPVI with localization of R38. R38 and other important residues for collagen binding, such as K59, K41, and R166,6 are localized on the extracellular domain of GPVI as crystallized by Horii et al.8 The 2 disulfide bridges are represented and encircled in black.
Characterization of the patient's GPVI molecular defect. (A) Flow cytometry on whole blood using the monoclonal antibody 3J24.2 showed decreased GPVI expression on the patient's platelets, compared with control. (B) Serial quantities of proteins in sodium dodecyl sulfate–platelet lysate from the patient or a control were immunoblotted with a human polyclonal anti-GPVI (hpAb). GPVI of the control migrated as a single 58-kDa band, whereas the patient's GPVI migrated as a smear and presented a partial deficiency (note that the 10-μg control GPVI band is saturated; the 35-kDa band is nonspecific). When 10 μg control and patient platelet proteins were immunoblotted with monoclonal antibody 3J24.2 or 9O12.2, the patient's GPVI was hardly detectable. (C) DNA sequence showing the single nucleotide substitution in exon 3 (C172T), resulting in the Arg38 substitution in Cys. (D) Purified recombinant wild-type (WT) and R38C GPVI-Fc were analyzed on sodium dodecyl sulfate–8% acrylamide gels and Coomassie blue staining. WT GPVI-Fc migrated as an approximately 220-kDa band and R38C GPVI-Fc as a smear in nonreducing conditions (NR), and both migrated as a major 95-kDa band after reduction with 5% β-mercaptoethanol (R). (E) Binding of WT and R38C GPVI-Fc to immobilized collagen. Results represent mean ± SD of 2 experiments performed in triplicate. The Kd values are 1.67 ± 0.26 μg/mL and 1.4 ± 0.17 μg/mL for R38C and WT GPVI-Fc, respectively. (F) Three-dimensional structure of the extracellular domain of GPVI with localization of R38. R38 and other important residues for collagen binding, such as K59, K41, and R166,6 are localized on the extracellular domain of GPVI as crystallized by Horii et al.8 The 2 disulfide bridges are represented and encircled in black.
Sequencing genomic GPVI DNA evidenced 2 sequence abnormalities: (1) a heterozygous single nucleotide substitution in exon 3 (C172T), resulting in the missense mutation arginine 38 to cysteine (Figure 2C); and (2) a heterozygous duplication of 5 nucleotides c356-360 (AGCCC) in exon 4, resulting in a frame shift and the appearance of a nonsense codon, 30 residues downstream (supplemental Figure 2). Sequencing of the cDNA confirmed that these 2 mutations were on different alleles: all transcripts contained the exon 3 C172T mutation, and none contained the exon 4 duplication, probably because of degradation through the nonsense mRNA decay mechanism.17 Thus, all the GPVI copies on the patient's platelets bore the R38C mutation.
Analysis of the parent's DNA showed that the mother is heterozygous for the C172T substitution in exon 3; the father is heterozygous for the 5 nucleotide duplication in exon 4, and his platelets exhibited a 50% GPVI deficiency but with a normal migration and subnormal responses to collagen (data not shown).
We hypothesized that the R38C mutation caused abnormal GPVI folding and function and introduced the mutation into recombinant soluble GPVI-Fc18 seen as a 210-kDa band in nonreducing conditions; the mutant protein migrated as a smear, reminiscent of the patient's profile (Figure 2D). Migration returned to normal after reduction, confirming that C38 forms mismatched disulfide bridges with only a small fraction of the protein correctly folded. R38C GPVI-Fc binding to collagen was markedly decreased (Figure 2E) to less than 25% of WT GPVI-Fc values, with a subnormal affinity, suggesting that only correctly folded GPVI binds collagen. Assuming a 50% expression of R38C GPVI on platelets, the number of functional copies should not exceed 10%, which is not sufficient for effective receptor clustering and downstream signaling. The binding of monoclonal antibodies to R38C GPVI-Fc was impaired (not shown), explaining why GPVI was less detectable on the patient's platelets.
Our observation confirms the importance of the integrity of the first Ig-like loop in the interaction of GPVI with collagen. Examination of GPVI tridimensional structure shows that R38, localized in the first Ig-like loop of the extracellular domain, interacts with Y66 and K41 (Figure 2F). These interactions stabilize the loop, which contains elements of the collagen-binding site, such as K41 and K59.6,19 The model also shows that a cysteine in this position could lead to the formation of aberrant disulfide bridges.
The only other patient reported with a congenital GPVI abnormality20 carried a heterozygous out-of-frame 16-bp deletion in exon 3. However, this patient presented a more severe bleeding phenotype, suggesting the possibility of an additional and not yet identified platelet defect. As previous observations of GPVI-deficient mice treated by aspirin showed an increased bleeding tendency,21 we suggest that the fairly good clinical outcome may become impaired by additional acquired risk factors for bleeding, such as hemostasis-interfering drugs. Our findings provide evidence that genetic abnormalities of GPVI, although rare, should be sought for in patients with a mild bleeding tendency.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Bernadette Boval for antiplatelet antibody analysis, Jean-Jacques Lacapère for structural analysis, Deborah François for R38C construction, Véronique Arocas for helpful discussion, and Mary Osborne-Pellegrin for editing the manuscript.
This work was supported by funds from Inserm, University Paris 7, Fondation de France (grant 200701960), and Bettencourt-Schueller Foundation.
Authorship
Contribution: B.D. performed experiments and wrote the manuscript; D.L. and C.R. identified and followed the patient; M.B. and V.O. performed experiments; C.O. and B.G. designed and conducted the genetic study; N.A. designed and supervised the study; and M.J.-P. designed and supervised the study and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Martine Jandrot-Perrus, U698 Inserm, Hôpital Bichat, 46 rue Henri-Huchard, 75877 Paris cedex 18, France; e-mail: Martine.Jandrot-Perrus@inserm.fr.