To the editor:
Hereditary severe congenital neutropenia (SCN) represents a heterogeneous group of diseases characterized by early-onset life-threatening bacterial infections associated with absolute neutrophil counts (ANC) below 500/μL.1,2 In recent years, different studies have identified germ-line, disease-causing mutations in ELA2, HAX1, GFI1, GCSF3, and WAS genes, which account for the different autosomal-dominant, autosomal-recessive, and X-linked forms of hereditary SCN.2-8 Recently, Boztug et al described biallelic G6PC3 mutations, encoding the catalytic subunit 3 of glucose-6-phosphatase, as disease-causing mutations in a type of hereditary SCN, often associated with cardiac and/or urogenital malformations.9
Here we report the clinical case of a young male Moroccan patient with a syndromic form of SCN resembling that recently described by Boztug et al.9 The patient is a 22-year-old male, fifth son of apparently nonconsanguineous parents, with no family history of the disease (Figure 1A). Since early childhood, he had suffered recurrent bacterial lung infections, which led to bronchiectasis, and severe gingivitis with the loss of several teeth. During his first decade of life, he failed to receive appropriate medical attention and was first referred to us at the age of 11 when he immigrated to Spain. Physical examination revealed a young, undernourished patient with active bacterial infections (orbit cellulitis), with height and weight below percentile 3 and educational delay. Blood analyses detected persistent decreased absolute neutrophil count (50-540/μL) and anemia (Hb 9.5 g/dL), although the latter resolved with iron supplementation. Bone marrow examination revealed a paucity of granulocyte series cells beyond the promyelocyte stage. Bilateral cryptorchidism, an unusual and prominent subcutaneous venous circulation, and an atrial septal defect were detected by physical examination and echocardiography (Figure 1B). A diagnosis of SCN was made, and treatment with recombinant human granulocyte colony-stimulating factor (rhG-CSF) at 5 μg/kg of body weight every 48 hours was started. A successful response was observed in the following months with an increase in absolute neutrophil count to 1000 to 1500/μL and a decrease in infection severity and frequency. The patient is currently receiving rhG-CSF at 7.5 μg/kg of body weight every 48 hours. During follow-up, intermittent and unexplained thrombocytopenia was observed, whereas no clonal hematopoietic disorder was detected.
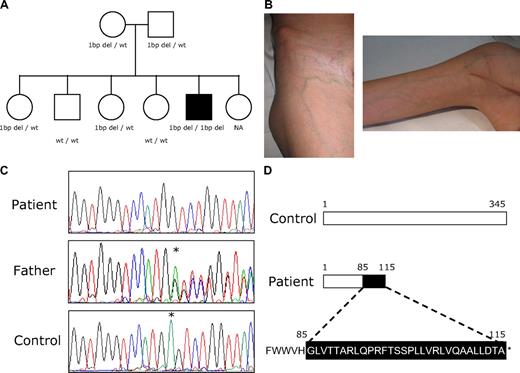
Clinical case. (A) Patient's family tree. The ■ denotes the patient with documented syndromic SCN; and open symbols, unaffected persons. G6PC3 genotype is shown below each symbol. NA indicates not available. (B) Prominent subcutaneous venous circulation detected in the patient. (C) Sequence electropherograms of G6PC3 gene showing the homozygous 1-bp deletion (patient, top line), heterozygous 1-bp deletion (patient's parents, middle line), and wild-type (healthy control, bottom line) genotypes. *Nucleotide position where the 1-bp deletion was detected. (D) Graphic of wild-type G6PC3 protein and predicted changes provoked by the novel homozygous 1-bp deletion detected in the patient.
Clinical case. (A) Patient's family tree. The ■ denotes the patient with documented syndromic SCN; and open symbols, unaffected persons. G6PC3 genotype is shown below each symbol. NA indicates not available. (B) Prominent subcutaneous venous circulation detected in the patient. (C) Sequence electropherograms of G6PC3 gene showing the homozygous 1-bp deletion (patient, top line), heterozygous 1-bp deletion (patient's parents, middle line), and wild-type (healthy control, bottom line) genotypes. *Nucleotide position where the 1-bp deletion was detected. (D) Graphic of wild-type G6PC3 protein and predicted changes provoked by the novel homozygous 1-bp deletion detected in the patient.
Sequencing analyses of different SCN-associated genes were performed, after the patient had provided written informed consent. No disease-causing mutations were identified in ELA2, HAX1 and WAS genes, whereas a homozygous 1-bp deletion was detected in exon 2 of G6PC3 gene, resulting in a frameshift at codon 85 and a premature stop codon at 115 (Figure 1C-D). We conclude that this novel G6PC3 variant is a new disease-causing mutation, not a benign polymorphism, on the basis of its detection in a patient with a syndromic form of SCN, its absence among 120 healthy controls and the clear phenotype-genotype segregation observed in this family, consistent with an autosomal-recessive inheritance pattern.
Authorship
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Cristina Díaz de Heredia, MD, Servicio de Hematología y Oncología Pediátrica, Hospital Infantil Vall d'Hebron, Paseo Vall d'Hebron, 119-129, 08035-Barcelona, Spain; e-mail: crdiaz@vhebron.net.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal