Abstract
The megakaryocytic (MK) and erythroid lineages are tightly associated during differentiation and are generated from a bipotent megakaryocyte-erythroid progenitor (MEP). In the mouse, a primitive MEP has been demonstrated in the yolk sac. In human, it is not known whether the primitive MK and erythroid lineages are generated from a common progenitor or independently. Using hematopoietic differentiation of human embryonic stem cells on the OP9 cell line, we identified a primitive MEP in a subset of cells coexpressing glycophorin A (GPA) and CD41 from day 9 to day 12 of coculturing. This MEP differentiates into primitive erythroid (GPA+CD41−) and MK (GPA−CD41+) lineages. In contrast to erythropoietin (EPO)–dependent definitive hematopoiesis, KIT was not detected during erythroid differentiation. A molecular signature for the commitment and differentiation toward both the erythroid and MK lineages was detected by assessing expression of transcription factors, thrombopoietin receptor (MPL) and erythropoietin receptor (EPOR). We showed an inverse correlation between FLI1 and both KLF1 and EPOR during primitive erythroid and MK differentiation, similar to definitive hematopoiesis. This novel MEP differentiation system may allow an in-depth exploration of the molecular bases of erythroid and MK commitment and differentiation.
Introduction
Although erythrocytes and platelets are mature cells with quite different functions, the erythroid and megakaryocytic (MK) lineages are closely related. Their similarities are observed at all stages of differentiation and include cytology, regulation by growth factors, and at the transcriptional level. Their homeostatic regulation is controlled by 2 humoral growth factors, erythropoietin (EPO) and thrombopoietin (TPO), which share strong structural homology and may derive from an ancestral gene.1,2 Moreover, the erythropoietin receptor (EPOR) and thrombopoietin receptor (MPL) are closely related. They are homodimeric class I cytokine receptors, and both require JAK2 for signaling.3,4 Numerous transcription factors, such as GATA2, GATA1, FOG1, TAL1/SCL, GFI1B, and NFE2, are critical for both erythroid and MK development,5-7 whereas others are more dedicated for unilineage differentiation, such as the erythroid EKLF (KLF1)8,9 and MYB10 or the MK FLI1,11 GABPA,12 and AML1.13
Studies of adult hematopoiesis, both in human and mice, have shown that the erythroid and MK lineages arise from a bipotential megakaryocyte-erythroid progenitor (MEP),14-16 a separate entity with no lymphoid and granulomonocytic potential.17 The precise hierarchical relationship between the MEP and other progenitors is controversial because it has been suggested that MEP may directly arise from a multipotent hematopoietic stem cell.18 The erythroid and MK lineages are also tightly associated during development as a primitive MEP has been detected in the mouse yolk sac.19 Because of limited availability of embryonic samples collected before the onset of definitive hematopoiesis, it is not known whether a MEP also exists during human primitive hematopoiesis. One way to overcome these limitations has been to use human embryonic stem cells (hES) to model in vitro the very early developmental stages of hematopoiesis.20 Indeed, it is now possible to efficiently produce erythroid and MK cells from hES cells.21-25
In the present study, we demonstrate that the primitive erythroid and MK lineages arise from a common MEP bipotent progenitor. Expression profiling allowed us to identify the gene expression signature of this progenitor and of its descendants, thus establishing a novel model for the study of erythroid and MK differentiation.
Methods
Cell lines and culture media
H1 (National Institutes of Health code WA01) and H9 hES (National Institutes of Health code WA09) cell lines were obtained from WiCell Research Institute. Most experiments were performed with the H1 cell line. Cells were maintained in an undifferentiated state on mouse embryonic fibroblasts in hES medium consisting of Dulbecco modified Eagle/F12 medium supplemented with 20% knockout serum replacement, 0.1 mM minimum essential medium nonessential amino acids solution, 2 mM l-glutamine, 50 U/mL penicillin, 50 μg/mL streptomycin (all from Invitrogen), 0.1 mM β-mercaptoethanol (Sigma-Aldrich), and 10 ng/mL human basic fibroblast growth factor (PeproTech). For this study, H1 and H9 hES 3 cells were used from passages 25 to 60. The mouse OP9 (Riken Institute, Osaka University, Osaka, Japan), and mouse MS5 stromal cell lines were used as feeder layers.
Antibodies and growth factors
Antibodies and their suppliers are provided in supplemental Table 1 (available on the Blood website; see the Supplemental Materials link at the top of the online article). Recombinant human hematopoietic cytokines were used at the following concentrations: EPO, 2 U/mL (OrthoBiotech); interleukin-3 (IL-3), 100 U/mL (generous gift from Novartis); stem cell factor (SCF), 50 ng/mL (generous gift from Amgen); and TPO, 50 ng/mL (generous gift from Kirin Laboratories).
hES differentiation on OP9 cells
To induce differentiation, hES cells were dissociated mechanically and seeded as small clumps onto a confluent OP9 stromal cell layer, previously treated with mitomycin C (Sigma-Aldrich) for 3 hours. Cells were cultured in minimum essential medium containing 20% hES-tested fetal bovine serum (FBS; Hyclone Laboratories). Medium was changed every 3 to 4 days. Growth factors were added at day 7 at indicated concentrations. At different time points, suspension and adherent cell fractions of the coculture were harvested by treatment with 0.05% trypsin-ethylenediaminetetraacetic acid solution (Invitrogen) for 3 minutes and subjected to cell sorting and functional and molecular assays (supplemental Figure 1).
Flow cytometry and cell sorting
For cell-surface marker identification, single-cell suspension containing both OP9 and hES cells was incubated for 30 minutes at 4°C with the different conjugated monoclonal antibodies (mAbs) and then washed twice. Cells were stained with 7-actinomycin D (7-AAD; Sigma-Aldrich) and immediately analyzed with a FACSort flow cytometer (BD Biosciences). For cell sorting, cell populations at different stages of differentiation (between 9 and 14 days of coculture) were sorted on a FACSDiva cell sorter (BD Biosciences). DNA content of hES-derived MKs was measured by propidium iodide staining after 9 to 16 days of hES/OP9 coculturing.
Quantification of hematopoietic progenitors in semisolid cultures
Sorted cell populations were plated in triplicate at a density of 1 to 3 × 103 cells/mL in human methylcellulose medium H4434 (StemCell Technologies). Progenitor-derived hematopoietic colonies were scored after 9 to 14 days. Alternatively, sorted cells from different populations were grown at a density of 1 to 3 × 103 cells/mL in serum-free fibrin clots in the presence of the following cytokines: TPO (10 ng/mL), SCF (50 ng/mL), IL-3 (10 IU/mL), and EPO (1 U/mL).14 MK colonies were scored after 5 to 7 days by indirect immuno-alkaline phosphatase labeling using an anti-GPIb mAb (anti-CD42a; clone GN287) or anti-GPIIb mAb.
Single-cell assay for combined erythroid and megakaryocytic potential
The MS5 cells were cultured for 24 hours to confluence on 96 flat microwell plates. After 12 days, GPA+CD41+ cells were sorted from hES/OP9 cocultures and seeded on an MS5 feeder layer at 1 cell per well in medium containing hES-tested FBS and a combination of SCF, EPO, TPO, and IL-3. After 5 days, proliferating clones were identified under an inverted microscope; at day 7 and day 12, cells were labeled with directly conjugated antiglycophorin A (GPA) and anti-CD41 mAbs and analyzed by flow cytometry.
Immunocytochemistry
Cultured MKs were layered on poly-L-lysine–coated slides (Menzel-Glaser) for 1 hour. Cells were then fixed with 2% paraformaldehyde for 5 minutes, permeabilized with 0.1% Triton X-100, and incubated with a rabbit anti–von Willebrand factor (VWF) polyclonal antibody (Tebu) for 1 hour. After 3 washes, cells were incubated with the appropriate secondary antibodies and then stained with 4,6 diamidino-2-phenylindole.
Cytospin preparation and cytologic staining
Sorted cell populations were centrifuged onto slides for 3 minutes at 500g (Cytospin II; Thermo). Smears were stained with May-Grünwald-Giemsa.
RNA isolation, RT-PCR, and quantitative RT-PCR
Total RNA was extracted with QIAGEN columns according to the manufacturer's recommendations. RNA quantification was assessed with NanoDrop Nd-1000 UV/Vis (Labtech France). A total of 1 μg RNA was reverse transcribed into cDNA as previously described.26 The resulting synthesized cDNA was amplified using the TaqMan Universal PCR Master Mix (Applied Biosystems) containing the specific primers (1.2 μM) and probe (0.1 μM). Quantitative polymerase chain reaction (PCR) was performed on a 7500 Real-Time PCR System (Applied Biosystems) with standard cycling conditions. Expression levels of all the genes were normalized to the expression of the TATA box-binding protein (TBP), scored as one of the most stable genes by GeNorm analysis.27 Quantification was done with the Pfaffl method, taking into account the amplification efficiencies of each primer gene couple.28 Relative expression ratios were calculated by reporting values to one of the tested conditions. Primers and probes were those publicly available on the RTPrimerDB primer database (http://medgen.ugent.be/rtprimerdb/) or were derived from an in-house in silico design.
Single-cell quantitative real-time PCR
Cells generated from hES/OP9 cocultures were sorted according to their distinctive expression of hematopoietic antigens and directly collected as single cell in a PCR plate containing 9 μL reverse-transcribed (RT) mixture as previously described26 but without RNase inhibitors and RT enzymes. Plates were spun to ascertain deposition of single-cell drops into the RT mixture, incubated for 2 minutes at 80°C, and cooled on ice. cDNA synthesis was performed by addition of RNase inhibitors and RT enzymes in each well (24 IU/well RNase OUT and 50 IU/well Superscript II, Invitrogen), and incubation during 2 hours at 50°C. The reaction was stopped by thermal heating for 15 minutes at 70°C. Final volume for all reverse transcription reactions was 10 μL. cDNA (2 μL) was analyzed in the 7500 Real-Time PCR System. Absolute quantification of each cDNA species was performed by dilution series of purified amplicons (QIAGEN), concentrations of which were measured using the NanoDrop.
Results
Simultaneous generation of erythrocytes and megakaryocytes bearing features of primitive hematopoiesis by coculture of human embryonic stem cells with OP9 cells
To induce the development of the erythroid and MK lineages, we used a 2-step protocol in which undifferentiated H1 or H9 hES cells were cocultured with OP9 stromal cells29,30 (supplemental Figure 1). In the first step, hES cells were grown for 7 days without growth factors, before adding a cocktail of cytokines (EPO, TPO, SCF, and IL-3). Using this experimental protocol, erythroid and MK differentiation (Figures 1–2) was efficiently achieved between day 9 and day 14 of coculture. This differentiation preceded the principal wave of myelopoiesis that occurred after day 14 (supplemental Figure 2) as previously reported for murine and hES cell differentiation.31,32
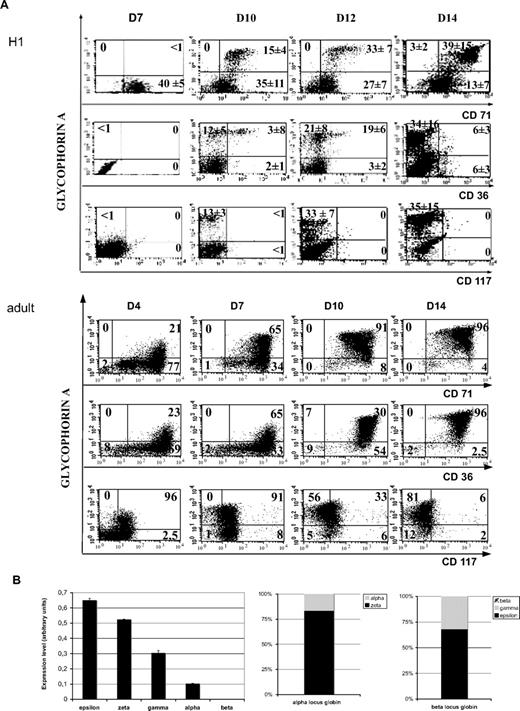
Coculture of human H1 hES cells with the OP9 stromal cell line results in simultaneous generation of mature erythroid and megakaryocytic cells. (A) Kinetics of erythroid maturation studied by flow cytometry. Embryonic (top panel) and adult (bottom panel) erythroid cells, obtained from the differentiation of mobilized CD34+ cells, cultured in the presence of SCF, EPO, and IL-3, were analyzed for the expression of GPA, CD36, and CD71 erythroid differentiation markers. The cells were also incubated with isotype-matched irrelevant antibodies as negative controls. 7-AAD–positive cells were excluded from the analyses by appropriate gating. Figure shows representative diagrams of 3 repeated determinations derived from H1 ES cell line. Values in each quadrant are the mean ± SD of 3 independent experiments. (B) Globin chains expression in hES-derived erythrocytic cells. On the left, histograms show mRNA expression of globin chains obtained using the quantitative PCR assay performed in triplicate. The error bars represent SD. The level of globin chain expression is illustrated. On the right, results are presented with respect to their gene locus. Bars represent expression levels of indicated transcripts relative to the overall expression of the α- or the β-gene locus.
Coculture of human H1 hES cells with the OP9 stromal cell line results in simultaneous generation of mature erythroid and megakaryocytic cells. (A) Kinetics of erythroid maturation studied by flow cytometry. Embryonic (top panel) and adult (bottom panel) erythroid cells, obtained from the differentiation of mobilized CD34+ cells, cultured in the presence of SCF, EPO, and IL-3, were analyzed for the expression of GPA, CD36, and CD71 erythroid differentiation markers. The cells were also incubated with isotype-matched irrelevant antibodies as negative controls. 7-AAD–positive cells were excluded from the analyses by appropriate gating. Figure shows representative diagrams of 3 repeated determinations derived from H1 ES cell line. Values in each quadrant are the mean ± SD of 3 independent experiments. (B) Globin chains expression in hES-derived erythrocytic cells. On the left, histograms show mRNA expression of globin chains obtained using the quantitative PCR assay performed in triplicate. The error bars represent SD. The level of globin chain expression is illustrated. On the right, results are presented with respect to their gene locus. Bars represent expression levels of indicated transcripts relative to the overall expression of the α- or the β-gene locus.
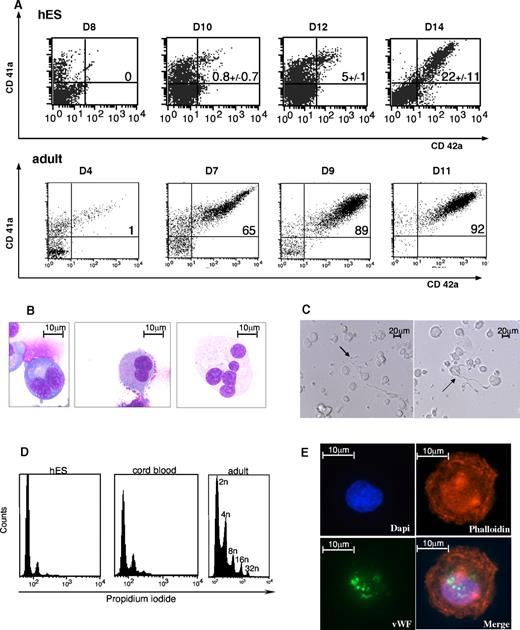
MK maturation derived from H9 hES cell. (A) CD41 and CD42 flow cytometry analysis. Expression of CD41 and CD42 MK differentiation markers, shown, respectively on the y-axis and x-axis of the dot plot, were analyzed in embryonic (left) and adult (right) megakaryocytic cells. As previously described in Figure 1A, the cells were incubated with isotype-matched irrelevant antibodies as negative controls, and dead cells were excluded from the analyses. Representative histograms and values in each quadrant are the mean ± SD of 3 independent experiments. (B) May-Grünwald-Giemsa-stained cytospins of CD41+CD42+ subsets isolated from a hES/OP9 coculture from day 14 to day 16. Various levels of maturation were observed in immature MK (first on the left) compared with mature MK (last on the right). (C) Light microscopy image of proplatelet formation in serum-free cultures. (D) Immunofluorescent staining of an MK with anti-VWF polyclonal antibody (green) and phalloidin (red) showed a granular pattern of labeling for VWF. MKs were left to adhere on polylysine-coated slides for immunolabeling and detection of cytoskeletal components. The images were acquired using a Zeiss laser scanning microscope (LSM 510; Carl Zeiss) or an inverted Leica DM IFBE microscope (Leica Microsystems) with a 63×/1.0 NA oil objective. (E) Ploidy distribution was measured by flow cytometry after staining hES-derived MKs with propidium iodide from day 14 to 16, CD34+ cord blood–derived MKs at day 10, and CD34+ leukapheresis-derived MKs at day 10.
MK maturation derived from H9 hES cell. (A) CD41 and CD42 flow cytometry analysis. Expression of CD41 and CD42 MK differentiation markers, shown, respectively on the y-axis and x-axis of the dot plot, were analyzed in embryonic (left) and adult (right) megakaryocytic cells. As previously described in Figure 1A, the cells were incubated with isotype-matched irrelevant antibodies as negative controls, and dead cells were excluded from the analyses. Representative histograms and values in each quadrant are the mean ± SD of 3 independent experiments. (B) May-Grünwald-Giemsa-stained cytospins of CD41+CD42+ subsets isolated from a hES/OP9 coculture from day 14 to day 16. Various levels of maturation were observed in immature MK (first on the left) compared with mature MK (last on the right). (C) Light microscopy image of proplatelet formation in serum-free cultures. (D) Immunofluorescent staining of an MK with anti-VWF polyclonal antibody (green) and phalloidin (red) showed a granular pattern of labeling for VWF. MKs were left to adhere on polylysine-coated slides for immunolabeling and detection of cytoskeletal components. The images were acquired using a Zeiss laser scanning microscope (LSM 510; Carl Zeiss) or an inverted Leica DM IFBE microscope (Leica Microsystems) with a 63×/1.0 NA oil objective. (E) Ploidy distribution was measured by flow cytometry after staining hES-derived MKs with propidium iodide from day 14 to 16, CD34+ cord blood–derived MKs at day 10, and CD34+ leukapheresis-derived MKs at day 10.
Next, we determined the maturation stages of erythroid and MK cells during time. Expression of GPA progressively increased from the first day of culture, whereas the level of CD71 was constantly high. A decrease in the expression of CD36 was observed from day 12 and thereafter. Thus, we identified a GPAhighCD71highCD36−/low cell subset by day 14 (Figure 1A). KIT was near the threshold of detection in contrast to a high level detected in erythroid cells differentiating from mobilized adult CD34+ cells. May-Grünwald-Giemsa staining of GPA+CD71+ cells at day 14 showed large-sized erythroblasts at different stages of maturation, including basophilic, polychromatophilic, and orthochromatic erythroblasts (supplemental Figure 3 top panel). Enucleated cells were not observed, consistent with primitive erythropoiesis. Globin detection by immunologic staining showed that the totality of the cells was positive for the Σ chain. The γ chain was detected only in a minority of cells, whereas β globin chain was undetectable (supplemental Figure 3 bottom panel). RT-PCR analysis detected all the globin chains tested, but not the β chain even in saturating conditions (supplemental Figure 4). Quantification by quantitative RT-PCR indicated that embryonic globin chains (Σ and ζ) were much more expressed than the fetal ones (γ and α; Figure 1B; 80% for ζ and 70% for Σ of the α- and β-globin loci, respectively).
MK maturation from hES cells was associated with an increased expression of CD41 and the appearance of CD42, leading to the presence of a CD41highCD42+ cell population, a phenotype defining mature MKs. However, CD42 membrane expression did not reach the high levels observed in adult MKs, and expression was at least 1 log lower (Figure 2A), although mature MKs were present (Figure 2B). Proplatelet formation (Figure 2C) was observed, albeit at a low rate. On 3 independent experiments, the mean percentage of the CD41highCD42+ cell population was 22% plus or minus 11% for the H9 cell line (Figure 2A). The CD41+CD42+ cell population also expressed VWF with a granular pattern (Figure 2D). Ploidy analysis of hES-derived MKs showed that they were of low ploidy levels with a maximum of 8N. In contrast, MKs derived from cord blood or adult CD34+ reached ploidy up to 16N and 32N, respectively (Figure 2E).
Taken together, our experimental protocol allowed the generation of erythroid cells and MKs with the characteristic hallmarks of primitive hematopoiesis.
The onset of E/MK differentiation is initiated with the development of a GPA+CD41+CD34+/−CD43+CD42− cell population
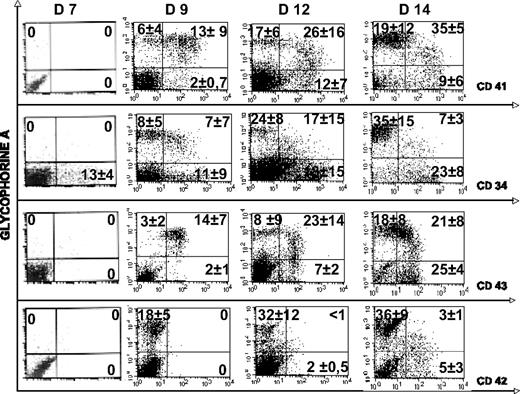
To further define the kinetics of erythroid and MK differentiation, we studied the expression of GPA and CD41 by double staining of H1 and H9 hES cells. The CD41 antigen (αIIb β3 integrin, platelet GPIIb/IIIa), considered to be restricted to the MK lineage, was found to be one of the most reliable markers of early steps of embryonic hematopoiesis in mice.33,34 A small percentage of cells coexpressing CD41 and GPA were detected at days 8 and 9 of differentiation, whereas single-positive cells (ie, GPA+CD41− or CD41+GPA− cells) were nearly absent. These double-positive cells, already described in cultures of ES cells,32 peaked at days 10 to 12 and reached maximum of approximately 50% of the cultured cells when the first erythroblasts and MKs were emerging (Figure 3). As the culture progressed, the frequency of double-positive cells declined, whereas the frequency of single-positive cells increased. Moreover, the double-positive cells expressed a lower intensity of GPA and a slightly higher intensity of CD41.
Kinetics of GPA, CD41, CD34, CD43, and CD42 expression during hematopoietic differentiation of H1 hES cells. Flow cytometric analysis of the expression of GPA (y-axis) and CD41, CD42, CD43, and CD34 (x-axis) was performed in hES cell differentiation culture. Plots represent the evolution of the respective proportions of different cell populations within the GPA-positive cells. The gates identifying cells expressing GPA alone or coexpressing CD41 and GPA were set to include only 7-AAD–negative cells. Gating was settled according the negative controls (not shown). Values in each quadrant are the mean ± SD of 3 independent experiments.
Kinetics of GPA, CD41, CD34, CD43, and CD42 expression during hematopoietic differentiation of H1 hES cells. Flow cytometric analysis of the expression of GPA (y-axis) and CD41, CD42, CD43, and CD34 (x-axis) was performed in hES cell differentiation culture. Plots represent the evolution of the respective proportions of different cell populations within the GPA-positive cells. The gates identifying cells expressing GPA alone or coexpressing CD41 and GPA were set to include only 7-AAD–negative cells. Gating was settled according the negative controls (not shown). Values in each quadrant are the mean ± SD of 3 independent experiments.
Additional phenotypic analysis of the GPA+CD41+ cells using the simultaneous detection of 4 antigens showed that these cells were positive for CD34 and CD43. In contrast, they did not express the CD45 antigen (data not shown). CD42 was not detected in this cell population except after day 12 (Figure 3).
These kinetic studies suggested that the CD41+GPA+ cell population (double-positive) was the precursor of the CD41−GPA+ and CD41+GPA− cell populations (single-positive) and could be a candidate cell population for an MEP.
MEP resides within the GPA+CD41+ cell population
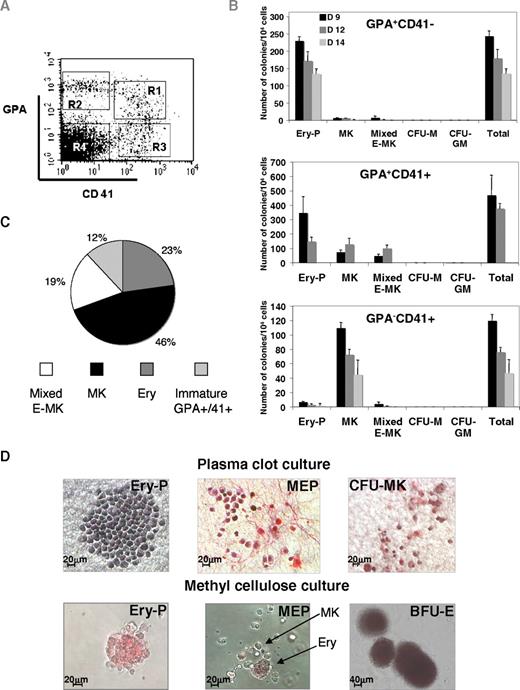
To test this hypothesis, we sorted the cells in 4 fractions: CD41+GPA+, CD41−GPA+, CD41+GPA−, and a double-negative CD41−GPA− cell population at different time points (Figure 4A). Among the sorted populations, the GPA+CD41+ cell fraction was the most enriched in progenitors with a cloning efficiency of approximately 5% in semisolid medium for the H1 cell line (Figure 4B). This fraction had a restricted erythro-MK potential (Figure 4B-C). Three main types of hematopoietic colonies were seen. Primitive erythroid colonies (Ery-P) grown in semisolid medium contained usually 10 to 100 large highly hemoglobinized erythroblasts (Figure 4D) at day 12 in semisolid medium. These colonies were morphologically distinguishable from erythroid burst-forming units (BFU-E) colonies derived from definitive erythropoiesis and found at later days of coculture (Figure 4D). MK colonies were identified after CD42 or CD41 staining in plasma clot cultures (Figure 4D). Colony-forming unit (CFU) MK-derived colonies were composed of a cluster of large cells (2-20 cells), which were subjected to rapid lysis after 5 to 7 days. These colonies differed from cord blood and adult CFU-MK–derived colonies by a low staining with the anti-CD42 mAb (Figure 4D; and data not shown). Mixed erythro-MK colonies had a distinguishable morphology. They had the appearance of Ery-P with the presence of a few large MKs, usually seen at the periphery (Figure 4D). These mixed colonies were still observed when cells were plated at low concentration (103 cells/mL), suggesting that they were not the result of the confluence of 2 colonies. These MEP-derived colonies represented, at day 12 of coculture, approximately one-third of the hematopoietic colonies. In contrast, the single-positive GPA+CD41− and GPA−CD41+ cell fractions were highly enriched in erythroid and MK progenitors, respectively, suggesting that the loss of GPA or CD41 is accompanied with a restriction in the colony-forming potential and a progressive commitment toward the MK or erythroid lineage. Mixed erythro/MK colonies were nearly absent from these 2 cell fractions (Figure 4B).
Differential expression of CD41 and GPA permits subfractionation of a compartment of primitive embryonic erythro-megakaryocytic progenitors. (A) Sorting strategy used to fractionate the erythro-MK progenitors. H1 hES cells were analyzed by flow cytometry after 12 days of differentiation. Cells within the CD41+ cell population were examined for GPA coexpression and subdivided into CD41+GPA+ (R1), CD41−GPA+ (R2), CD41+GPA− (R3), and CD41−GPA− (R4) cell fractions. (B) Colony-forming potential of the different GPA/CD41 cell populations derived from H1 hES cells. Cells sorted at day 9, 12, and 14 were seeded into fibrin clot culture and tested for their colony-forming potential. Cultures were allowed to grow for 7 to 10 days, fixed, and stained with an anti-CD42 antibody. Colonies were scored under an inverted microscope for Ery-P (erythroid colony), MK (megakaryocytic colony), mixed E-MK (bipotent erythroid/MK), CFU-M (macrophage colony), and CFU-GM (granulo-macrophagic colony). Data represent the mean value ± SD from 3 experiments. Although mixed E-MK colonies were present in all tested subpopulations, multivariate analysis of variance showed that the highest frequency was found within the GPA+CD41+ fraction (P < .001). (C) Analysis of the cell composition of indivdual clones derived from H9 GPA+CD41+ cells. GPA+CD41+ cells were sorted after 12 days from hES /OP9 cocultures and seeded on MS5 feeder layer at 1 cell per well, in medium containing hES-tested FBS and a combination of SCF, EPO, TPO, and IL-3. The results presented are those obtained from 75 clones analyzed at day 7 and day 12 of culture after labeling with directly conjugated anti-GPA and anti-CD41 monoclonal antibodies. (D) Semisolid assays. (Top panel) Immunostaining of fibrin clot colonies grown in fibrin clots in the presence of a cocktail of cytokines. The primitive erythroid colony (Ery-P), mixed erythro-megakaryocytic colony (MEP), and pure megakaryocytic colony (CFU-MK) were stained with an anti-CD41 Ab (pink). (Bottom panel) Methylcellulose cultures showing typical primitive erythroid colonies (Ery-P) and mixed E-MK colonies containing erythroblasts and megakaryocytes (MEP). Colonies with typical morphologic features of BFU-E obtained from the double-negative population are clearly different from Ery-P–derived colonies. Colonies were examined under a Zeiss laser scanning microscope (LSM 510; Carl Zeiss) equipped with a 63×/1.4 numerical aperture (NA) oil objective (original magnifications 20× and 10×; MEP in fibrin clot and BFU-E).
Differential expression of CD41 and GPA permits subfractionation of a compartment of primitive embryonic erythro-megakaryocytic progenitors. (A) Sorting strategy used to fractionate the erythro-MK progenitors. H1 hES cells were analyzed by flow cytometry after 12 days of differentiation. Cells within the CD41+ cell population were examined for GPA coexpression and subdivided into CD41+GPA+ (R1), CD41−GPA+ (R2), CD41+GPA− (R3), and CD41−GPA− (R4) cell fractions. (B) Colony-forming potential of the different GPA/CD41 cell populations derived from H1 hES cells. Cells sorted at day 9, 12, and 14 were seeded into fibrin clot culture and tested for their colony-forming potential. Cultures were allowed to grow for 7 to 10 days, fixed, and stained with an anti-CD42 antibody. Colonies were scored under an inverted microscope for Ery-P (erythroid colony), MK (megakaryocytic colony), mixed E-MK (bipotent erythroid/MK), CFU-M (macrophage colony), and CFU-GM (granulo-macrophagic colony). Data represent the mean value ± SD from 3 experiments. Although mixed E-MK colonies were present in all tested subpopulations, multivariate analysis of variance showed that the highest frequency was found within the GPA+CD41+ fraction (P < .001). (C) Analysis of the cell composition of indivdual clones derived from H9 GPA+CD41+ cells. GPA+CD41+ cells were sorted after 12 days from hES /OP9 cocultures and seeded on MS5 feeder layer at 1 cell per well, in medium containing hES-tested FBS and a combination of SCF, EPO, TPO, and IL-3. The results presented are those obtained from 75 clones analyzed at day 7 and day 12 of culture after labeling with directly conjugated anti-GPA and anti-CD41 monoclonal antibodies. (D) Semisolid assays. (Top panel) Immunostaining of fibrin clot colonies grown in fibrin clots in the presence of a cocktail of cytokines. The primitive erythroid colony (Ery-P), mixed erythro-megakaryocytic colony (MEP), and pure megakaryocytic colony (CFU-MK) were stained with an anti-CD41 Ab (pink). (Bottom panel) Methylcellulose cultures showing typical primitive erythroid colonies (Ery-P) and mixed E-MK colonies containing erythroblasts and megakaryocytes (MEP). Colonies with typical morphologic features of BFU-E obtained from the double-negative population are clearly different from Ery-P–derived colonies. Colonies were examined under a Zeiss laser scanning microscope (LSM 510; Carl Zeiss) equipped with a 63×/1.4 numerical aperture (NA) oil objective (original magnifications 20× and 10×; MEP in fibrin clot and BFU-E).
These 3 cell fractions were studied at different days of coculture ranging from day 9 to day 14. Enough cells were recovered in each cell fractions to perform clonal assays, except for the GPA+CD41+ cell fraction. No difference in the type of progenitors present in each fraction was observed during this kinetic, but their content in bipotent progenitors declined with time. Of note, despite IL-3 addition, no myeloid colony was observed. However, granulo-macrophage and BFU-E–derived colonies (Figure 4D) with a different pattern of globin chain expression were seen in the fractions depleted in GPA+ and CD41+ cells (supplemental Figure 4) at later days of coculture.
To further demonstrate the existence of an MEP, individual GPA+ CD41+ cells from H9 were grown in 96-well plates on a MS5 feeder in the presence of cytokines. Wells containing hematopoietic cells were identified after 6 days of culture under a microscope. Cells were labeled with anti-GPA and anti-CD41 antibodies and analyzed by flow cytometry. Four types of clones were observed: erythroid clones containing only GPA+CD41− cells, MK clones containing exclusively GPA−CD41+ cells, megakaryocyte/erythroid clones (MEP) containing both GPA+CD41− and GPA−CD41+ cells, and clones with still some GPA+ CD41+ cells representing progenitors that have not yet completely differentiated (Figure 4C). The MEP represented approximately one-fifth of the progenitor content of the GPA+CD41+ cell fraction, a proportion slightly lower than found in semisolid medium but with the H1 cell line.
These data establish a developmental hierarchy among erythroid and MK subsets derived from primitive hematopoiesis, with CD41+GPA+ cells being the most primitive subset exhibiting an erythro-MK differentiation potential, whereas GPA+CD41− and GPA−CD41+ cells represent more mature populations committed to either the erythroid or MK lineages.
Erythroid and megakaryocytic lineage-specific genes are coexpressed in embryonic MEP
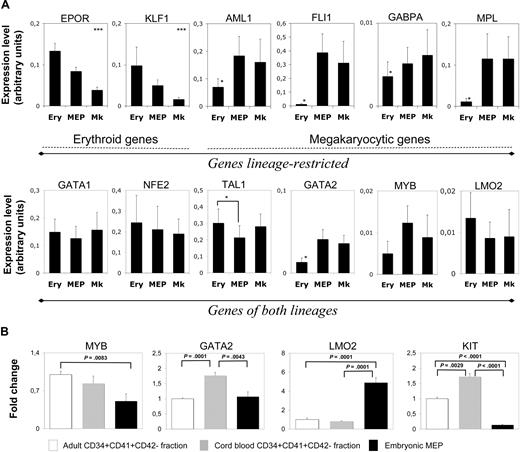
Having defined the hierarchy among these cell fractions, we studied whether differentiation could be traced to the molecular level. We investigated expression of 2 cytokine receptors (EPOR and MPL), 3 transcription factors involved in MK differentiation (AML1, FLI1, and GABPA), 1 involved in erythroid differentiation (KLF1), and 6 involved in both lineages (GATA1, NFE2, TAL1, GATA2, MYB, and LMO2), in H1 cells. GPA+CD41+, GPA+CD41−, and GPA−CD41+ cell fractions (called Ery, MEP, and MK, respectively, in Figure 5A) were sorted by flow cytometry (Figure 4A), and gene expression was analyzed by quantitative RT-PCR (Figure 5A). They show stringent differences in the erythroid and MK commitment from the MEPs derived from hES cells. MK differentiation was associated with no significant change in the expression of “MK” genes (AML1, FLI1, GABPA, and MPL), but by a down-regulation of “erythroid” genes (EPOR and KLF1). In contrast, erythroid differentiation was characterized by a down-regulation of “MK” genes together with an induction of “erythroid” genes. Among the genes associated with differentiation in both lineages, GATA1, NFE2, and LMO2 had similar expression levels at all stages of differentiation. The level of TAL1 slightly increased on differentiation, especially for the erythroid lineage. In contrast, the level of GATA2 was much higher in MEP and MK cell fractions than in the erythroid cell fraction, an expected finding as GATA2 plays a later role in MK than in erythroid differentiation. More surprisingly, MYB was detected during both differentiation stages with a tendency to be more expressed in MKs than in erythroid cells. Nonetheless, expression levels of GATA2, MYB, and also LMO2 were much lower at all differentiation stages compared with the other erythroid/MK genes. Considering the importance of these 3 genes in hematopoiesis, we investigated whether their expression could differ between primitive and definitive erythro/MK differentiation (Figure 5B). The level of LMO2 expression was higher in the embryonic MEP than in cord blood– or adult-derived CD34+CD41+CD42− fraction, a cell fraction highly enriched in MK progenitors but also containing low number of MEPs. In contrast, GATA2 and MYB levels were similar or close to that found in these adult or neonate cell fractions.
Embryonic MEPs coexpress erythroid and megakaryocytic genes. (A) Relative expression levels of 12 genes as assessed by quantitative RT-PCR. Genes were classified according to their restricted expression in the erythroid (EPOR and KLF1) or megakaryocytic (AML1, FLI1, GABPA, and MPL) lineages (top panel). The second group was composed of genes common to the 2 types of differentiation (GATA1, NFE2, TAL1, GATA2, MYB, and LMO2). Relative expression levels (fold change) of GPA+CD41+ (MEP) and GPA−CD41+ (MK) cells were reported to the expression levels measured in the GPA+CD41− (Ery) population. Values represent the mean ± SEM of at least 3 independent experiments. Details of the protocol for this analysis are specified in “Single-cell quantitative real-time PCR.” *Significant difference (P < .05). (B) Relative expression levels of MYB, GATA2, LMO2, and KIT transcripts in embryonic MEP versus cord blood and adult progenitor cells (CD34+CD41+CD42−). Values were reported to expression levels found in the adult CD34+CD41+CD42− fraction and represent the mean ± SEM of at least 3 independent experiments.
Embryonic MEPs coexpress erythroid and megakaryocytic genes. (A) Relative expression levels of 12 genes as assessed by quantitative RT-PCR. Genes were classified according to their restricted expression in the erythroid (EPOR and KLF1) or megakaryocytic (AML1, FLI1, GABPA, and MPL) lineages (top panel). The second group was composed of genes common to the 2 types of differentiation (GATA1, NFE2, TAL1, GATA2, MYB, and LMO2). Relative expression levels (fold change) of GPA+CD41+ (MEP) and GPA−CD41+ (MK) cells were reported to the expression levels measured in the GPA+CD41− (Ery) population. Values represent the mean ± SEM of at least 3 independent experiments. Details of the protocol for this analysis are specified in “Single-cell quantitative real-time PCR.” *Significant difference (P < .05). (B) Relative expression levels of MYB, GATA2, LMO2, and KIT transcripts in embryonic MEP versus cord blood and adult progenitor cells (CD34+CD41+CD42−). Values were reported to expression levels found in the adult CD34+CD41+CD42− fraction and represent the mean ± SEM of at least 3 independent experiments.
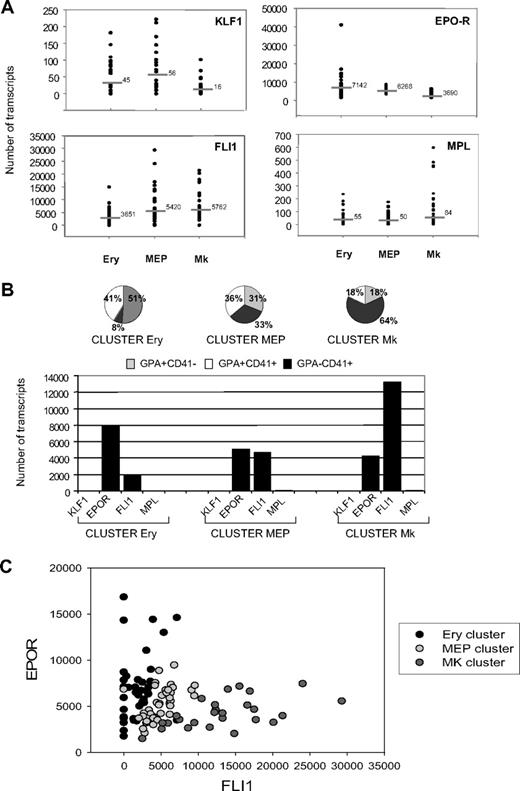
Single-cell PCR expression of FLI1, KLF1, EPOR, and MPL allows monitoring of the erythro/MK commitment
Figure 5 showed that expression of 2 pairs of genes (KLF1 and EPOR) and (FLI1 and MPL) was markedly inhibited during MK and erythroid differentiation, respectively (Figure 5A). Therefore, we sought to use these genes to molecularly trace commitment at the single-cell level, as each cellular subfraction remains heterogeneous. We analyzed the quantitative expression of these 4 genes in single GPA+CD41−, GPA+CD41+, and GPA−CD41+ cells derived from H1. Of a total of 132 wells containing single sorted cells, 108 were positive for GAPDH transcripts used as a control housekeeping gene. An average of 36 cells from each cell fraction were individually analyzed. The expression level of each gene transcript was plotted for the 3 cell fractions (Figure 6A). At the single-cell level, erythroid differentiation was associated with an increase in EPOR transcripts and a down-regulation of FLI1. Engagement to MK differentiation was associated with the up-regulation of MPL and a concomitant decrease of both EPOR and KLF1 (Figure 6A). To ascertain a possible correlation among the 4 genes in the various fractions, we calculated Pearson correlation coefficients between the genes in 2-by-2 comparisons (Table 1). As expected, the association of EPOR-KLF1 and MPL-FLI1 was positively correlated in the GPA+CD41− and GPA−CD41+ cell fractions, respectively. In the GPA+CD41+ cell fraction that coexpressed the 4 genes, we observed an opposite correlation between KLF1 and MPL genes (Table 1). In search of unique gene expression profiles, we ran K-means cluster analysis of expression of EPOR, MPL, KLF1, and FLI1. The totality of the transcript data obtained in the single-cell level was integrated to assess for clustering in specific pattern of expression independently of the cell fraction from which they were isolated. Three major patterns of expression were identified, which we named on the major subpopulation represented in the cluster: cluster Ery (51% GPA+CD41−), cluster MK (64% GPA−CD41+), and cluster MEP (31% GPA+CD41−, 36% GPA+CD41+, 33% GPA−CD41+; Figure 6B top panel). Cells with a MK or Ery cluster were rare (18% and 8%, respectively) in cells defined as erythroid (GPA+CD41−) or MK (GPA−CD41+) on their immunophenotype. In contrast, the MEP cluster included a similar proportion of cells from each fraction underscoring its heterogeneity. In these 3 clusters, the shift between the various phenotypes was merely governed by changes in the balance between EPOR and FLI1 (Figure 6B bottom panel). Nonetheless, the balance between EPOR and FLI1 and the loss of GPA or CD41 antigens were not totally coordinated, as attested by the presence of cells belonging to the MEP clusters among GPA+CD41− and GPA−CD41+ cells. Considering the expression of EPOR and FLI1 only, we could thus discriminate the various cell clusters that were clearly separated (Figure 6C).
Expression of EPOR and FLI1 discriminates the progressive commitment of progenitor cells in the GPA+CD41+ cell fraction. (A) Scatter plot indicating transcript levels for KLF1, EPOR, FLI1, and MPL in Ery, MEP, and MK fractions. Each dot represents the absolute number of transcripts present in each cell. Horizontal bars represent the average levels for each gene in the various populations calculated considering the log normal distribution of the data (Shapiro-Wilk normality test run to confirm that the transcript distribution is log-normal at 95% significance level; P = .05). The absolute number of transcripts for each gene was retrieved by comparing threshold cycle values of the cDNAs to those obtained from a dilution series of a well-defined quantity of a purified PCR product. (B) Unsupervised cluster analysis (K-means) of expression of EPOR, MPL, KLF1, and FLI1 in Ery, MEP, and MK fractions at a single-cell level. The gene transcript values of the 4 genes in the overall fractions were integrated together to assess for independent clustering in specific patter of expression. Three clusters were identified: cluster Ery, cluster MEP, and cluster MK (top panel). Names were given according to the main cell type represented in each cluster (cluster Ery with 52% of cells GPA+CD41−, cluster MK with 64% of cells GPA−CD41+). The cluster MEP was equally represented by the GPA+CD41−, GPA+CD41+, and GPA−CD41+ cell subsets. Profiles of gene expression were derived to describe each individual cluster (bottom panel). (C) Dot plot distribution between EPOR and FLI1 at a single-cell level. Spots from Ery, MEP, and MK clusters were colored differently. Each spot represents a single cell. Here, 3 clouds of points are observed, where cells in the MEP cluster progressively commit to the Ery or MK cluster by increasing the number of copies of EPOR or FLI1 transcripts, respectively.
Expression of EPOR and FLI1 discriminates the progressive commitment of progenitor cells in the GPA+CD41+ cell fraction. (A) Scatter plot indicating transcript levels for KLF1, EPOR, FLI1, and MPL in Ery, MEP, and MK fractions. Each dot represents the absolute number of transcripts present in each cell. Horizontal bars represent the average levels for each gene in the various populations calculated considering the log normal distribution of the data (Shapiro-Wilk normality test run to confirm that the transcript distribution is log-normal at 95% significance level; P = .05). The absolute number of transcripts for each gene was retrieved by comparing threshold cycle values of the cDNAs to those obtained from a dilution series of a well-defined quantity of a purified PCR product. (B) Unsupervised cluster analysis (K-means) of expression of EPOR, MPL, KLF1, and FLI1 in Ery, MEP, and MK fractions at a single-cell level. The gene transcript values of the 4 genes in the overall fractions were integrated together to assess for independent clustering in specific patter of expression. Three clusters were identified: cluster Ery, cluster MEP, and cluster MK (top panel). Names were given according to the main cell type represented in each cluster (cluster Ery with 52% of cells GPA+CD41−, cluster MK with 64% of cells GPA−CD41+). The cluster MEP was equally represented by the GPA+CD41−, GPA+CD41+, and GPA−CD41+ cell subsets. Profiles of gene expression were derived to describe each individual cluster (bottom panel). (C) Dot plot distribution between EPOR and FLI1 at a single-cell level. Spots from Ery, MEP, and MK clusters were colored differently. Each spot represents a single cell. Here, 3 clouds of points are observed, where cells in the MEP cluster progressively commit to the Ery or MK cluster by increasing the number of copies of EPOR or FLI1 transcripts, respectively.
Pearson correlations of EPOR, MPL, KLF1, and FLI1 in erythroid, MEP, and megakaryocytic cells
| . | EPOR . | MPL . | KLF1 . | FLI1 . |
|---|---|---|---|---|
| Ery (GPA+CD41−) | ||||
| EPOR | 1 | |||
| MPL | −0.04 | 1 | ||
| KLF1 | 0.46* | 0.08 | 1 | |
| FLI1 | 0.05 | 0.13 | 0.14 | 1 |
| MEP (GPA+CD41+) | ||||
| EPOR | 1 | |||
| MPL | −0.03 | 1 | ||
| KLF1 | 0.01 | −0.39* | 1 | |
| FLI1 | −0.11 | 0.02 | −0.08 | 1 |
| MK (GPA−CD41+) | ||||
| EPOR | 1 | |||
| MPL | 0.19 | 1 | ||
| KLF1 | 0.13 | 0.11 | 1 | |
| FLI1 | 0.15 | 0.34* | 0.04 | 1 |
| . | EPOR . | MPL . | KLF1 . | FLI1 . |
|---|---|---|---|---|
| Ery (GPA+CD41−) | ||||
| EPOR | 1 | |||
| MPL | −0.04 | 1 | ||
| KLF1 | 0.46* | 0.08 | 1 | |
| FLI1 | 0.05 | 0.13 | 0.14 | 1 |
| MEP (GPA+CD41+) | ||||
| EPOR | 1 | |||
| MPL | −0.03 | 1 | ||
| KLF1 | 0.01 | −0.39* | 1 | |
| FLI1 | −0.11 | 0.02 | −0.08 | 1 |
| MK (GPA−CD41+) | ||||
| EPOR | 1 | |||
| MPL | 0.19 | 1 | ||
| KLF1 | 0.13 | 0.11 | 1 | |
| FLI1 | 0.15 | 0.34* | 0.04 | 1 |
The highest correlation found.
EPO is absolutely required for the primitive human embryonic erythropoiesis
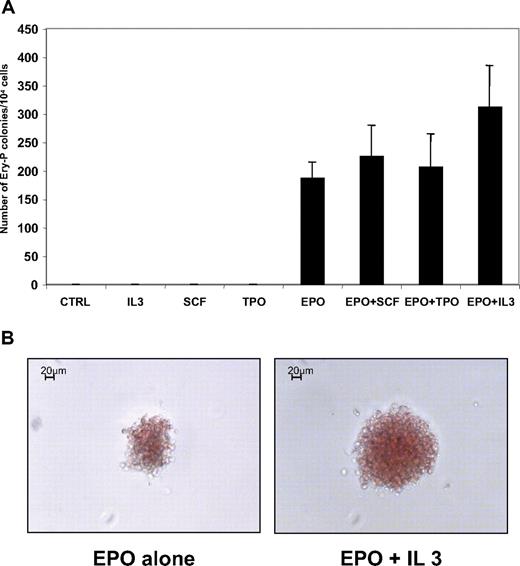
In the mouse, it has been suggested that EPO was not a requirement for primitive erythropoiesis.35-37 Thus, we evaluated the cytokine response of erythroid progenitors (GPA+CD41−) purified from H1 hESC/OP9 coculture at day 12. As shown in Figure 7A, EPO was absolutely required for Ery-P formation, and its effect could not be replaced by SCF, IL-3, or TPO, as described in the mouse. SCF, IL-3, and TPO had no synergy with EPO on the plating efficiency of Ery-P. However, a slight synergy was observed between EPO and IL-3 and, to a lesser extent, between EPO and SCF, leading to an increase in colony size, which could contain up to 200 hemoglobinized cells (Figure 7B). These “large” Ery-P colonies represented approximately 25% of the colonies growing in presence of EPO and IL-3.
Erythropoietin is absolutely required for human primitive embryonic erythropoiesis. (A) Methylcellulose colony assay in various growth factor combinations. GPA+CD41+ cells were sorted at day 12 of hES/OP9 coculture and tested for colony-forming activity in the presence of different combinations of cytokines, as indicated. Cytokines were used at the following concentrations: EPO, 2 U/mL; TPO, 50 ng/mL; SCF, 50 ng/mL; and IL-3, 100 U/mL. Colonies were scored at day 9 of culture. The mean ± SD of 3 experiments is presented. (B) Morphology of Ery-P colonies grown with EPO alone (left) or with EPO + IL-3 (right). Note the difference in colony sizes.
Erythropoietin is absolutely required for human primitive embryonic erythropoiesis. (A) Methylcellulose colony assay in various growth factor combinations. GPA+CD41+ cells were sorted at day 12 of hES/OP9 coculture and tested for colony-forming activity in the presence of different combinations of cytokines, as indicated. Cytokines were used at the following concentrations: EPO, 2 U/mL; TPO, 50 ng/mL; SCF, 50 ng/mL; and IL-3, 100 U/mL. Colonies were scored at day 9 of culture. The mean ± SD of 3 experiments is presented. (B) Morphology of Ery-P colonies grown with EPO alone (left) or with EPO + IL-3 (right). Note the difference in colony sizes.
Discussion
Human adult erythropoiesis and megakaryopoiesis share a common bipotential progenitor, the MEP,14-16 although there is evidence that MK differentiation might occur by alternative pathways.17 In the mouse, it has recently been shown that 2 waves of MEPs occur in the yolk sac during development: one belonging to primitive hematopoiesis and the second to definitive hematopoiesis.19 In human hematopoiesis, MEPs derived from definitive hematopoiesis have been already described, but primitive MEPs have not been characterized. To investigate the primitive erythroid and MK differentiation, we have used the hematopoietic differentiation of hES cells that recapitulates human ontogeny.
A previous study has shown that coculture of hES cells on OP9 stromal cells for 4 to 5 days induced a hematopoietic differentiation phenotypically defined by the expression of the CD43 antigen.32 Among the CD43+ cell population, CD41+ and GPA+ cells were restricted to the MK and erythroid lineages.32 We have extended these results by demonstrating that the erythroid and MK differentiation, which occurred from day 8 to day 14 of coculture, was confined to 3 cell populations: GPA+CD41−, GPA−CD41+, and GPA+CD41+. The GPA+CD41− and GPA−CD41+ cell populations contained nearly exclusively erythroid and MK progenitors, respectively. The double-positive GPA+CD41+ cell population contained erythroid and MK progenitors but also a significant proportion (20%) of progenitors, giving rise to mixed colonies composed of erythroblasts and MKs but devoid of macrophages. We confirmed the existence of a MEP by performing single-cell cultures. In addition, this approach validated that the double-positive cells (GPA+CD41+) were at the origin of single-positive cells (GPA+CD41− or GPA−CD41+). The human erythroid/MK differentiation derived from hES cells cocultured on OP9 for 8 to 12 days exhibited several criteria of primitive hematopoiesis. Erythroblasts were large nucleated cells that synthesized embryonic hemoglobins and MKs were hypoploid, a characteristic of yolk sac MKs in the mouse.19,35 In addition, hES-derived MKs expressed a low level of GPIb (CD42), thus differing from neonate or adult MKs. The primitive human and murine MEPs share many similarities and give rise to small colonies composed of less than 100 erythroid cells and a few MKs at the periphery of the colony. Thus, colonies derived from Ery-P or MEP have similar appearance except for the presence of a few MKs in MEP. However, the main difference between human and mouse hematopoiesis appears to be an absolute EPO requirement of the primitive human hematopoiesis.36-38
Overall, the phenotype of primitive human MEPs differs from definitive MEPs17 because the cell coexpresses differentiation markers associated with terminal erythroid (GPA) and MK (CD41) differentiation and undergoes accelerated differentiation. In adult mouse, a bipotential erythroid/megakaryocytic precursor also coexpressing glycophorin and platelet glycoproteins16 has been described in the bone marrow or spleen after an erythroid stress.39 Thus, primitive hematopoiesis and stress erythropoiesis, which both are associated with an accelerated production of erythrocytes, may have some similarities at the level of hematopoietic progenitors.
In definitive hematopoiesis, the mechanisms of MEP commitment have not yet been completely identified. There is evidence that the competition between KLF1 and FLI1 on one hand8,9,40 and the expression level of MYB41,42 on the other hand regulate the MEP fate, and that several other transcription factors such as GATA1, GATA2, and NFE2 are necessary for both erythroid and MK differentiation.5-7 Our study demonstrates that a distinct pattern of transcription factors and cytokine receptors is associated with the 3 cellular subsets that were analyzed. When the 3 global populations were considered, the progressive commitment was evidenced by a decrease in EPOR/KLF1 gene expression and an increase in AML1 and GABPA only in MK cells, whereas commitment to the erythroid lineage was associated with a decrease in MPL/FLI1/AML1/GABPA/GATA2 gene expression and an increase in EPOR/KLF1 gene expression. Interestingly, MK commitment of the MEP was associated with a decrease of the 2 erythroid-specific genes (EPOR and KLF1) but without the induction of MK genes (FLI1, AML1, GABPA, and MPL). In contrast, erythroid commitment of the MEP was ascertained by both an increase in erythroid gene expression and a decrease in MK gene expression.
One limitation of the approach relied in the cell heterogeneity of each population. This is particularly true for the MEP-enriched population, which contains committed erythroid and MK progenitors. Thus, expression of FLI1, KLF1, EPOR, and MPL transcripts were investigated at the unicellular level by quantitative techniques. We could depict a well-ordered hierarchy, at cellular and molecular levels, from bipotential to monopotential progenitor cells. This hierarchy was particularly well defined by the inverse relationship between FLI1 and the EPOR transcript levels. The schematic model of MEP differentiation toward erythroid and MK lineages is illustrated in supplemental Figure 5.
The expression level of MYB transcripts was quite surprising because levels in MEP, MK, and erythroid cell fractions were similar, even with a decreasing trend in erythroid cells. However, the overall level of MYB expression was low but quite similar to that observed in adult or neonate progenitor. In the mouse, it has been demonstrated that MYB plays an important role in the commitment toward the erythroid lineage for the definitive hematopoiesis and, in contrast, that MYB was not necessary for primitive erythro/MK differentiation.43 However, recent studies strongly suggest that the MYB protein level is posttranscriptionally regulated by mIR150 in human42 ; therefore, the level of MYB transcript might not correlate with the protein level. Further experiments will be required to understand whether such a process occurs in primitive hematopoiesis. In contrast, LMO2 was expressed 5-fold higher in primitive MEP than in the fetal or adult progenitors. Considering the high level of TAL1 expression in MEP and erythro/MK primitive cells, the LMO2/TAL1 complex44,45 might have an important role during primitive hematopoiesis, a hypothesis awaiting additional investigation.
In conclusion, the results presented herein provide the first evidence for the presence of a common megakaryocyte-erythroid progenitor in the primitive human embryonic hematopoiesis. These findings suggest that the close association between the erythroid and megakaryocytic lineages is conserved throughout the ontogeny of the human hematopoietic system. The model proposed in our study might be useful to dissect the mechanisms underlying erythroid and MK commitment as well as the differences between primitive and definitive hematopoiesis.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank F. Wendling, S. Constantinescu, and C. Marty for critical reading of the manuscript; Kirin Brewery (Tokyo, Japan) for the gift of recombinant human thrombopoietin; M. Bengtsson and M. Kubista for advice in single-cell quantitative PCR assays and continuous assistance during the initial phases of the technique; H. Moniz, L. Chaussumier, and A. Magniez for their help and advice with hES cultures; S. Badaoui and L. Lordier for erythro-megakaryocytic cultures; P. Gonin for statistical analyses; and O. Wagner-Ballon for cytologic pictures.
This work was supported by grants from Médicen Paris région (consortium Ingecell) and Inserm. O.K. was supported by a fellowship from the Ministère de la Recherche. M.M. was supported by Inserm. T.L. was supported by Médicen.
Authorship
Contribution: O.K. and M.M. designed and performed experiments, analyzed data, and wrote the paper; M.M. developed single-cell quantitative PCR on hES; A.D.S. and T.L. performed experiments; F.L. performed sorting experiments and analyzed data; Y.L. performed sorting experiments; O.F. had the hES cell lines in charge; W.V. designed the research and wrote the paper; and F.N. and N.D. designed and supervised the experiments, and wrote the paper.
The current address of Dr Mori is Institute of Tropical Medicine (ITM), Antwerp, Belgium.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Najet Debili, Inserm, U790, Institut Gustave Roussy, 39 rue Camille Desmoulins, Villejuif, 94805, France; e-mail: denali@igr.fr.
References
Author notes
*O.K. and M.M. contributed equally to this study.
†F.N. and N.D. contributed equally to this study.