Abstract
Effective gene therapy requires robust delivery of the desired genes into the relevant target cells, long-term gene expression, and minimal risks of secondary effects. The development of efficient and safe nonviral vectors would greatly facilitate clinical gene therapy studies. However, nonviral gene transfer approaches typically result in only limited stable gene transfer efficiencies in most primary cells. The use of nonviral gene delivery approaches in conjunction with the latest generation transposon technology based on Sleeping Beauty (SB) or piggyBac transposons may potentially overcome some of these limitations. In particular, a large-scale genetic screen in mammalian cells yielded a novel hyperactive SB transposase, resulting in robust and stable gene marking in vivo after hematopoietic reconstitution with CD34+ hematopoietic stem/progenitor cells in mouse models. Moreover, the first-in-man clinical trial has recently been approved to use redirected T cells engineered with SB for gene therapy of B-cell lymphoma. Finally, induced pluripotent stem cells could be generated after genetic reprogramming with piggyBac transposons encoding reprogramming factors. These recent developments underscore the emerging potential of transposons in gene therapy applications and induced pluripotent stem generation for regenerative medicine.
Introduction
Convincing evidence continues to emerge from clinical trials that gene therapy is effective to treat patients with a wide range of diseases, resulting in long-term therapeutic effects.1-4 In particular, in children with otherwise lethal hereditary disorders as a result of congenital immune deficiencies, long-term clinical benefits have been obtained after gene therapy, and these children are now essentially leading normal lives. Despite these successes, gene therapy has also faced a number of setbacks, and there have been concerns about the safety of some gene delivery approaches. Although multiple factors appear to determine the success of gene therapy, the major limiting mechanisms identified today are insertional mutagenesis by integrating viral vectors, inappropriate expression of the transgene in unwanted cell types or conditions, and immune activation against the viral vector, the gene-engineered cells, or the transgene product.5-7 The consequences of these events range from the failure to establish stable transgene expression or elimination of the gene-modified cells to the triggering of acute systemic toxicity because of immune reactions and even transformed cell growth and oncogenesis.8 Some of these side effects are inherent to the type of viral vectors used. Moreover, production of viral vectors for clinical trials has been fraught with technical and regulatory hurdles. Hence, there is a need to develop safe and efficient alternatives based on nonviral vectors, which are less immunogenic than viral vectors. Unfortunately, most nonviral vectors are not capable of achieving high and stable expression of the therapeutic gene. However, the use of nonviral gene delivery approaches in conjunction with the latest generation transposon technology may now potentially overcome some of these limitations, which is the focus of this review.
Transposons for gene transfer
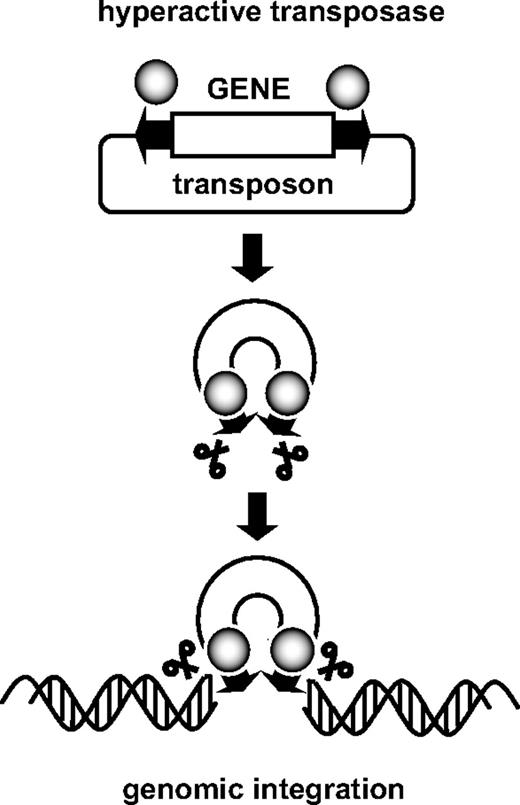
Transposons are discrete elements of DNA that have the distinctive ability to move from one chromosomal location to another. Transposons fall into 2 major categories: (1) retrotransposons that undergo transposition via an RNA intermediate and (2) DNA transposons that move directly as DNA. Transposition of this latter class involves a conservative cut-and-paste mechanism, in which the transposon gets excised from the donor locus and is subsequently integrated into another location by the transposase protein. This process has a key feature that makes DNA transposons particularly attractive as gene delivery tools. Namely, the transposase can act in trans on virtually any DNA sequence that is flanked by the terminal repeat sequences, normally found at each end of the transposon. Consequently, to turn DNA transposons into a gene delivery tool, a binary system has been developed (Figure 1) composed of (1) an expression plasmid that encodes the transposase and (2) a donor plasmid containing the DNA to be integrated, which is flanked in cis by the transposon terminal repeat sequences required for transposition. The transposase binds these terminal repeat sequences and catalyzes the excision of the gene of interest from the donor plasmid as well as its insertion into the genome of the host cell. By physically separating the transposase gene from the transposon-containing donor plasmid, it is possible to optimize the stoichiometry of both components. However, the transposase gene can also be placed on the same plasmid as the transposon (but located outside the terminal repeats). On the basis of their remarkable ability to integrate into the target cell genome, DNA transposons have been developed as tools for insertional mutagenesis and germline transgenesis in invertebrates (reviewed in Mates et al9 ). Although several Tc1/mariner transposons isolated from insects and nematodes turned out to have some activity in vertebrates, none of these heterologous elements were sufficiently robust in mammalian cells, which hampered their use for gene therapy.
Schematic representation of the transposition reaction. The transposase binds the terminal repeats (arrows) flanking the gene of interest and catalyzes the excision of the transposon and its subsequent genomic integration.
Schematic representation of the transposition reaction. The transposase binds the terminal repeats (arrows) flanking the gene of interest and catalyzes the excision of the transposon and its subsequent genomic integration.
To obtain a clinically relevant transposon that could be used for gene therapy, it was important to identify transposable elements, capable of efficient transposition in mammalian cells. A first step in this direction was achieved by molecular reconstruction of a synthetic, active Tc1/mariner-type transposon called Sleeping Beauty (SB). Until now, 3 transposon systems have the potential to be developed into a gene therapy vector: SB, Tol2, and piggyBac (PB).
Sleeping Beauty
SB was assembled by combining fragments of silent and defective Tc1/mariner elements from salmonid fish, and probably resemble an ancestral transposon that had become inactivated during evolution.10 The reconstructed SB showed appreciable transposition efficiencies in vertebrate cells that, at the time, were higher than any other transposon tested. In particular, the originally rederived SB transposon was at least 10-fold more efficient than other Tc1/mariner transposons.11 One limitation of transposons of the mariner family, including SB, is that transposition efficiency decreases in the presence of an excess of transposase, a phenomenon termed “overproduction inhibition.” Hence, the transposon-to-transposase ratio needs to be optimized.
The resurrection of SB has had broad implications for functional genomics, insertional mutagenesis, transgenesis, and somatic gene therapy.9,12-15 Although this was an important milestone in the field, the original “resurrected” SB was still not sufficiently robust for human gene therapy applications.16 Thus, enhancing transpositional activity is one of the main challenges for transposon vector development. In an attempt to increase its activity, almost every single amino acid had been changed in the transposase by “importing” amino acids and small blocks of amino acids from related transposases,17-19 systematic alanine-scanning,20 and rational replacement of selected amino acid residues.17 Together, these studies have yielded approximately 15 single-amino-acid replacements, each resulting in a relatively modest increase in transpositional activities. Most recently, we have derived novel engineered transposases from SB with the use of a high-throughput, in vitro molecular evolution, and selection paradigm21 that involved the following steps: first, hyperactive SB transposases were generated by incorporating phylogenetically conserved amino acids from related transposases belonging to the Tc1/mariner transposon family into SB; subsequently, by using a high-throughput, polymerase chain reaction–based, DNA-shuffling strategy, a library of mutant transposase genes was established to identify pairwise, synergistic combinations of these hyperactive mutations; finally, in an analytical approach, some of these hyperactivating mutations identified in the screen were combined. The most hyperactive version, hereafter referred to as SB100X, contained 6 combinatorial units that yield nearly 4 500 000 possible combinations, underscoring the necessity of combining “high-throughput” and “analytical” strategies. This SB100X version was greater than 100-fold more potent in HeLa cell lines compared with the originally resurrected SB in mobilizing a transposon that is integrated in the chromosome. The precise mechanism of the increased transposition efficiency of SB100X is not well understood, but it seems to be at least partly because of improved folding properties of the transposase that might increase transposase stability. However, a more detailed structural analysis of the protein–DNA complex would be necessary to fully understand the hyperactive nature of SB100X.
Tol2
Another recent addition to the transposon toolbox is Tol2. Tol2 is a naturally occurring, hAT superfamily fish transposon that shows activity in a wide range of vertebrate species, including human cells.22 One advantage of Tol2 is that it can transfer genes of up to 11 kb with minimal loss of transposition activity.23 Thus, the Tol2 transposon vector has the potential to carry fairly large DNA inserts, in comparison with the possible size limitation observed in a Tc1/mariner-type transposon. Tol2 transposition is directly proportional to the level of transposase and thus does not appear to exhibit overproduction inhibition.24 Tol2 creates single-copy insertions and does not cause gross rearrangements around the integration sites. However, disadvantages of Tol2 are the relatively low transposition activity compared with PB and hyperactive SB systems, and that, similar to other hAT transposons and PB,25 Tol2 might have a preference for insertion into genes, thereby presenting a safety issue (see “Safety issues”). Moreover, modifications at its N-terminus essentially abolishes Tol2 transposition,24 indicating that it will be challenging to generate a site-specific integrating Tol2 transposase by molecular engineering for targeted integration into chromosomal “safe harbors” (see “Safety issues”).
PiggyBac
PB was first identified when it underwent transposition from its insect host, the cabbage looper moth Trichoplusia ni, into the baculovirus genome.26 Recently, it was shown that PB could catalyze transposition in human and mouse somatic cells.25,27 When comparing side by side the transposition efficiency of PB with that of an early-generation SB transposase and Tol2 in different cell lines, PB consistently showed higher activity.24 However, subsequent comparison between PB and the latest hyperactive SB100X version showed that SB100X is more active in different cell lines and primary cells (see “Ex vivo gene delivery”).21,28 To further boost the efficiency of PB transposition, PB expression levels were increased by codon-optimization strategies, which did not alter the amino acid composition of PB.29 However, it may be worthwhile to increase PB activity per se, using similar molecular evolution schemes that successfully yielded hyperactive SB versions. SB and PB are complementary transposon systems, and it would seem premature to decide which transposon system would be preferred, because their properties and relative efficacies may vary depending on the application and target cells, the size of the insert, or the ability to alter their targeting specificity.
Gene therapy applications that use transposons
The use of transposons may overcome some of the manufacturing and regulatory hurdles intrinsic to the production of viral vectors. Nonviral vectors can be assembled in cell-free systems from well-defined components and have the potential to be less immunogenic than viral vectors. This may facilitate clinical applications and result in relatively low-cost nonviral vector production. In contrast, viral vector production depends on packaging cells. Another advantage of using transposons for gene therapy is that relatively large transgenes can easily be accommodated in SB or PB vectors. This would open new perspectives for gene delivery of large (> 10 kb) therapeutic transgenes, which cannot be packaged in most viral vectors. Although the transposition efficiency decreases with increasing insert size,30 inserts of up to 10 kb can be delivered with the use of SB transposons. However, when the transgene is flanked by 2 complete SB elements (so-called “sandwich” configuration), transposition efficiencies can be significantly improved.17 It has been shown that PB can efficiently transpose inserts of up to 14 kb.31 In contrast, the genetic design of most viral vectors is restricted because of intrinsic structural and size constraints. In particular, γ-retroviral and lentiviral vectors have an intrinsic packaging limit of less than 10 kb, which is even lower in the case of adeno-associated virus (< 5 kb).
Ex vivo gene delivery
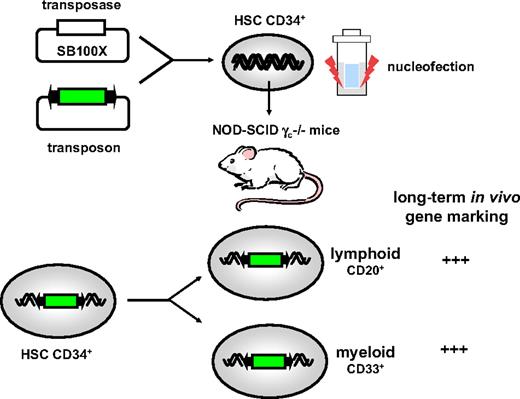
Transposon and transposase constructs can be delivered to the target cells by transfection with carrier molecules that facilitate their entry into the target cells. Some of the transfection methods such as electroporation, nucleofection, magnetofection had all been used for ex vivo gene delivery of transposon/transposase constructs, resulting in long-term and efficient transgene expression. In the absence of stable gene integration, expression from plasmid-based vectors typically declines in the days after transfection, especially in dividing cells. One of the first demonstrations that transposons could be used to correct patient's cells was obtained by transfecting keratinocytes from patients with junctional epidermolysis bullosa with a transposon encoding laminin along with a selectable marker gene.32 The corrected cells were selected and able to regenerate human skin on immunodeficient mice. Notably, over the past 20 years, it has been particularly challenging to develop an efficient, nonviral gene delivery system to genetically modify hematopoietic stem cells (HSCs). Only γ-retroviral, lentiviral, and foamy viral vectors were sufficiently robust to achieve stable gene transfer into HSCs at efficiencies that were clinically relevant.1,2,33-35 However, we have recently demonstrated that efficient and stable nonviral gene transfer into bona fide HSCs or progenitor stem cells could be achieved by nonviral means.21 To achieve this, CD34+ HSCs were enriched from cord blood and transfected by nucleofection with a transposon encoding a marker gene and a plasmid expressing the latest generation hyperactive SB100X transposase (Figure 2). Up to 40% of the hematopoietic colonies expressed the reporter gene (GFP) encoded by the SB transposon from a CMV/β-actin promoter (CAG).21 In contrast, fewer GFP+ colonies could be obtained with the early-generation SB or PB transposases, consistent with earlier reports,36 underscoring the superior transposition efficiency of SB100X. The transfected CD34+ cells retained their ability to differentiate in vitro along distinct lymphohematopoietic lineages. Most importantly, gene-marked cells were able to functionally reconstitute myeloablated immunodeficient recipient mice after transplantation with the CD34+ HSCs that had been transfected with the transposon/SB100X constructs. Interestingly, common integration sites were found in both the myeloid and lymphoid lineages, indicating that transposition had probably occurred in bona fide HSCs or progenitor stem cells capable of hematopoietic reconstitution. To our knowledge, this was the first demonstration that transposons can be used for efficient gene marking in vivo after hematopoietic reconstitution with transfected HSCs. This was later confirmed in an independent study.28 Here also, the CAG promoter was used to drive the reporter gene in the transposon construct. The availability of these novel hyperactive transposases may greatly facilitate clinical implementation of ex vivo gene therapy based on the nonviral genetic modification of HSCs for the treatment of hematopoietic disorders and cancer. Consequently, this new generation of hyperactive transposons may affect the current ex vivo stem cell–based gene delivery approaches that are being considered for clinical trials. It will now be essential to prove that this novel hyperactive transposon system can be used to achieve phenotypic correction in patients' cells or in animal models that mimic the cognate human hematopoietic disease, including primary immunodeficiencies. Our initial proof-of-concept study21 and the subsequent confirmatory study,28 now pave the way toward the use of lineage-specific promoters (ie, megakaryocyte-specific GpIIb promoter, erythroid-specific β-globin promoter) for directed transgene expression in specific hematopoietic lineages.
Transposons for HSC gene transfer. Plasmid encoding the hyperactive SB100X transposase were cotransfected by nucleofection with transposon-containing constructs in CD34+ stem or progenitor cells enriched from cord blood. Transfected cells were subsequently injected into immunodeficient NOD-γc−/− (nonobese diabetic–severe combined immunodeficient) mice. The transplanted cells contributed to efficient hematopoietic reconstitution and robust multilineage gene marking.21
Transposons for HSC gene transfer. Plasmid encoding the hyperactive SB100X transposase were cotransfected by nucleofection with transposon-containing constructs in CD34+ stem or progenitor cells enriched from cord blood. Transfected cells were subsequently injected into immunodeficient NOD-γc−/− (nonobese diabetic–severe combined immunodeficient) mice. The transplanted cells contributed to efficient hematopoietic reconstitution and robust multilineage gene marking.21
In vivo gene delivery
Direct in vivo gene delivery of transposon/transposase constructs had been achieved by polyethylenimine (PEI) into the lungs37 or brain tumors.38 PEI-based systemic administration of SB transposons encoding endothelial nitric oxide synthase from a cytomegalovirus (CMV) promoter resulted in endothelial nitric oxide synthase expression in pulmonary endothelial cells, leading to inhibition of induced pulmonary hypertension in rats.39 Similarly, intravenous injection of SB-expressing factor VIII from an elongation factor 1α promoter complexed to PEI resulted in therapeutic factor VIII levels and correction of the bleeding diathesis in hemophilic factor VIII–deficient mice.40 SB transposons encoding antiangiogenic genes that formed a complex with PEI were shown to inhibit angiogenesis and tumor growth in human glioblastoma xenografts, resulting in improved mouse survival.38
Alternatively, transposon/transposase constructs can be delivered by hydrodynamic injection to transfect hepatocytes in mouse models.41 Transgene expression levels and duration of expression vary, depending on the type of promoter used and the efficiency of transposition. Hepatocyte-specific promoters, such as the α1-antitrypin promoter, in conjunction with the apolipoprotein E hepatocyte control region,21,42 typically results in more prolonged expression at higher levels, compared with when ubiquitously expressed viral promoters are used (eg, CMV).16 Moreover, we have recently shown that the hyperactive SB100X transposase results in significantly higher stable levels of coagulation FIX (in the physiologic range) compared with when an early-generation SB transposase was used.21 In the absence of transposition, transgene expression typically declined in the course of several weeks, suggesting that extrachromosomal plasmids do not persist in transfected hepatocytes. This in vivo transposon gene delivery paradigm has been validated in different mouse models that mimic the cognate human disease, including hemophilia A and B,16,40,43 type I tyrosinemia,23,44 and mucopolysaccharidosis.45 Most of these studies relied on the use of ubiquitously expressed promoters such as CAG or elongation factor 1α to drive the therapeutic gene, but the use of a robust tissue-specific promoter would be preferred to exclude ectopic transgene expression in antigen-presenting cells and consequently minimize the risk of developing antibodies to the transgene product.
Hepatic transposition could be accomplished with SB and Tol2 transposon.23 Whether hydrodynamic transposon-mediated gene delivery could result in robust and safe stable gene expression in large animal models remains to be evaluated. Nevertheless, it is encouraging that hydrodynamic gene delivery can be achieved in liver lobes of pigs with the use of specially designed catheters, without causing any morbidity.46
Clinical applications
The Recombinant DNA Advisory Committee recently approved in 2008 the first-in-man gene therapy clinical trial that used transposons. In this trial, T cells genetically altered with the use of SB transposons will be transferred for adoptive immunotherapy into patients with CD19+ B lymphoid malignancies.47 To achieve this, SB transposons will be cotransfected by electroporation into T cells along with a construct expressing an early-generation hyperactive SB transposase. The transposon encodes a chimeric antigen receptor (CAR) that encompasses a CD19-specific mouse single-chain variable fragment linked to the CD28 endodomain fused with the CD3-ζ cytoplasmic domain. These transposon-modified T cells exhibit specific cytotoxicity toward CD19+ lymphoid tumors and significantly reduced tumor growth and improved survival after adoptive transfer in tumor-bearing mice.47 For the trial, the genetically modified T cells will be expanded on irradiated CD19+ tumor cell lines. Study subjects will receive full myeloablative chemotherapy, a peripheral blood stem cell autologous transplantation, and a monoclonal antibody to CD20 before infusion of the autologous genetically modified T cells during the period of chemotherapy- and antibody-induced lymphopenia. The study seeks to determine the feasibility, safety, and persistence of SB transposon-modified T cells in vivo. Previous studies that used CAR-modified T cells suffered from the relatively short duration of survival of manipulated T cells in vivo consistent with only modest measurable therapeutic results. However, the use of these new CARs in the SB trial are aimed at improving T-cell signaling and survival and may potentially overcome some of the limitations of earlier retargeting strategies. In any case, this clinical trial should provide useful information about the properties of SB-transfected cells in patients. Moreover, it may set the stage for new clinical trials based on transposons, including the most recently developed hyperactive versions.
Safety issues
Stable genomic integration is a particularly attractive feature to enable stable expression of the gene of interest dividing target cells and, in particular, in stem or progenitor cells and their differentiated progeny. Nevertheless, it is a double-edged sword that may potentially contribute to insertional oncogenesis and genotoxicity. Indeed, integration of a γ-retroviral vector encoding the interleukin-2 receptor common γ chain in proximity of the LMO2 protooncogene contributed to the deregulated LMO2 expression because of murine leukemia virus long terminal repeat (LTR).8 This ultimately accounted for the leukemogenesis observed in 4 subjects enrolled in a clinical trial for severe combined immune deficiency type X1.7 Moreover, γ-retroviral, lentiviral, and adeno-associated viral vectors show an integration bias into transcriptional units, indicating that integration is not random per se. γ-Retroviral vectors have a predilection toward integrating in the immediate proximity of transcription start sites and a small window around DNAse I hypersensitive sites, whereas lentiviral vectors are more likely to integrate further away from the transcription start site into active transcription units.48 This increases the risk of insertional oncogenesis compared with the use of vectors that do not exhibit such integration bias. Foamy virus vectors had a distinct integration profile compared with other types of retroviruses. Although integration was nonrandom, foamy viral vectors did not seem to integrate preferentially within genes, despite a modest preference for integration near transcription start sites and a significant preference for CpG islands.49 Transcriptional profiling showed that gene expression had little influence on integration site selection. When comparing γ-retroviral, lentiviral, and foamy viral vectors, γ-retroviral integrants showed the most significant frequency of occurrence very close (< 2.5 kb) to transcription start sites, but a substantial portion of all 3 retroviral integrants were within 50 kb.50 Importantly, γ-retroviral integrants were found more frequently in and near protooncogenes, suggesting this retroviral system may be the most prone to adverse gene activation.
In contrast, SB transposons do not appear to show an integration bias toward genes and instead exhibit a random pattern of integration.51,52 Interestingly, by incorporating SB into an integration-defective lentiviral vector and supplying the SB100X transposase in trans, it is possible to substitute the biased integration pattern of lentiviral vectors with that of SB.53 Hence, SB transposons may represent a safer alternative to the integrating viral vectors. Moreover, SB transposition is not associated with any recombination or deletion event at the integration sites. SB transposons integrate into a TA-dinucleotide that is duplicated on transposition. However, the random integration pattern of SB does not appear to be generally applicable to all transposon systems used in vertebrates. Indeed, PB does not exhibit a random genomic integration profile like SB but, instead, shows an integration pattern that resembles that of integrating viral vectors.25
Although SB shows no apparent biased integration into genes, intragenic integrations can still occur which carries an intrinsic genotoxic risk. However, in contrast to the murine leukemia virus LTR, the terminal repeat sequences of SB have very low intrinsic promoter/enhancer activity and consequently cannot readily activate endogenous genes that flank the transposon integration sites.54 Nevertheless, the internal promoter/enhancer used to drive the expression of the gene of interest may potentially activate expression of neighboring genes in proximity of the integration site. To address this concern, we recently showed that flanking the expression cassette in an SB transposon with insulator sequences reduced the risk of cis activation of neighboring genes.54 The same strategy could be applied to reduce the risk of insertional oncogenesis with other transposon-based gene delivery approaches.
Ideally, it would be desirable to establish targeted transposition into a safe harbor in the human genome. This could potentially be accomplished by engineering chimeric transposases fused to heterologous site-specific DNA-binding domains. However, one of the challenges of making such “designer” transposases lies in the fact that not all transposases tolerate N-terminal or C-terminal fusions. Nevertheless, N-terminal DNA-binding domain fusions with PB have been reported which do not compromise PB function, in contrast to N-terminal fusions with SB or Tol2.24 Thus, it appears that the PB transposase may be more permissive for this type of engineering than the other transposases. However, to overcome the apparent structural constraints of the SB system, it may be possible to use a molecular bridge that is able to influence target site determination of the SB transposase by noncovalent interactions. We have established proof of concept that this type of indirect retargeting is feasible with the use of the SB system.55 Despite these advances, one of the important challenges in the field is to show that designer transposases can result in robust and safe retargeting of genomic integration into safe chromosomal loci.
Another concern relates to the potential inadvertent genomic integration of the transposase-encoding construct. If the transposase is continuously expressed, it may result in uncontrolled transposition or “hopping” of the integrated transposons that may contribute to an increased genotoxic risk. Fortunately, however, plasmid integration is an inefficient process mediated by nonhomologous recombination that typically result with less than 10−5 stable integrants/transfected cells. Alternatively, the transposase can be provided as mRNA or protein.56 This modality obviates possible concerns about inadvertent stable integration of transposase-encoding plasmids in the target cells.
One concern that applies specifically to PB is that, in contrast to SB, which has no close relatives in the human genome, there are PB-like elements dispersed on different human chromosomes. Despite the evolutionary gap between man and moth, it cannot be excluded that an endogenous PB-like transposase could excise and integrate a transposon flanked with PB termini or, conversely, that an exogenous source of PB transposase may mobilize some endogenous human PB-like elements.57 This would need to be addressed further in anticipation of potential clinical applications.
Transposons and induced pluripotent stem cells
Induced pluripotent stem (iPS) cells are promising adult stem cells for regenerative medicine. iPS cells are derived from autologous somatic cells after genetic reprogramming and have first been described by Takahashi et al58,59 and later independently conformed by others60 (reviewed in Hochedlinger and Plath61 ). Typically, genetic reprogramming of mouse and human fibroblasts can be achieved after ectopic expression of a defined combination of 4 transcription factors, namely c-Myc, Klf4, Oct4, and Sox2. The main advantage of iPS cells is their remarkable pluripotency, which resembles that of embryonic stem cells. However, their derivation from human embryos raises some ethical concerns, and there are challenges about histoincompatibility barriers that would need to be addressed. In contrast, iPS cells can be obtained from autologous, histocompatible adult somatic cells, obviating the need for prolonged immunosuppressive therapy in the context of cell transplantation. iPS cells can be genetically modified and can be coaxed to differentiate into endodermal, mesodermal, and ectodermal cell types for transplantation to treat degenerative or genetic diseases. In one landmark study, iPS cells derived from fibroblasts of mice with sickle cell anemia were genetically corrected by replacing the mutant β-globin allele with a wild-type allele by homologous recombination. This provided a source of iPS cells able to differentiate into disease-free hematopoietic precursors that cured the afflicted mice after transplantation.62
Despite the robust gene transfer with γ-retroviral and lentiviral vectors, there are several reasons why an alternative approach would still be desirable for iPS induction. First, a specific risk associated with the use of γ-retroviral and lentiviral vectors for iPS generation relates to the ectopic expression of the delivered transcription factors in the progeny of the reprogrammed cells. Expression from γ-retroviral vectors is unpredictable and often depends on the differentiation status of the cells. In particular, methylation of the viral LTR was associated with transcriptional repression of the factors in iPS cells, whereas demethylation of the same sequences was associated with reactivation of expression of the factors in the iPS progeny. Because the c-Myc and Klf4 reprogramming factors are known oncogenes, their expression or reactivation in iPS-derived mice caused tumors.63 The safety of iPS cell derivation can potentially be improved by excluding c-Myc and Klf4 from the reprogramming cocktail63 or by selecting target cell types that already endogenously express these genes.64 Although these strategies show promise, they compromise reprogramming efficiency. Second, insertional mutagenesis mediated by γ-retroviral or lentiviral vectors may contribute to oncogenesis in the iPS-derived progeny. Third, although iPS cells have been generated by transient transfection of the reprogramming genes with nonviral vectors, the efficiency of iPS cell induction with nonviral vectors is rather modest and was unsuccessful in many primary human cell types.65,66
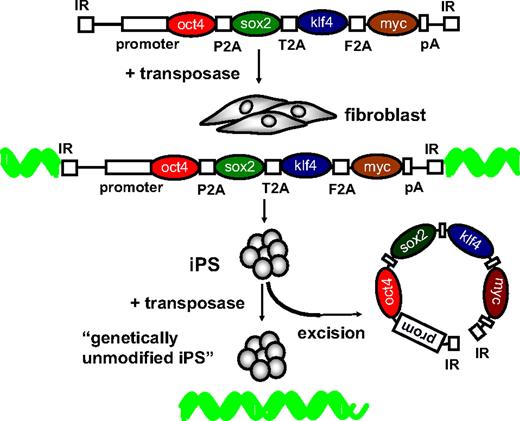
Several recent studies provide an alternative, more efficient, and safer strategy that involves viral vector-free integration of reprogramming genes, followed by their removal67-69 (Figure 3). This was achieved by first incorporating all 4 reprogramming genes into a single PB vector. The reprogramming genes were separated with a viral 2A oligopeptide. This allows for the posttranslational cleavage of the polypeptide, allowing synthesis of the 4 factors from a single transcript. Nonviral transfection into mouse or human fibroblasts resulted in the generation of bona fide iPS cells that could give rise to endodermal, mesodermal, and ectodermal lineages, consistent with what had been accomplished previously with γ-retroviral or lentiviral vectors. Nevertheless, to further validate the pluripotency of the nonviral mouse iPS cells, it would be necessary to generate iPS-derived offspring. The most important and unique feature of this approach is that reintroduction of the PB transposase by transient transfection resulted in the traceless excision of the reprogramming cassette from the iPS cell.69 This obviates most of the aforementioned concerns or limitations associated with the use of γ-retroviral, lentiviral, or nonintegrating vectors for iPS induction, while maintaining a relatively robust reprogramming efficiency. As an alternative to transposase-mediated excision, it was also possible to excise the reprogramming cassette flanked by loxP sites after transient expression of the CRE recombinase.68 However, in that case some residual trace elements outside the loxP sites, including the transposon repeats, cannot be excised from the iPS genome. These studies confirm the hypothesis that transient expression of the reprogramming factors suffices to convert somatic cells into iPS cells and underscore the important and broad implications of using transposons for reversible genetic reprogramming and iPS induction. This paves the way toward the generation of patient- or disease-specific iPS cells for regenerative medicine, provided some of the aforementioned concerns related to the endogenous PB-like sequences can be satisfactorily addressed. Alternatively, to overcome these PB-specific concerns, it may be worthwhile to explore the use of alternative transposons for iPS cell generation, including hyperactive SB100X.
Transposon-based reversible genetic modification as a novel paradigm for iPS induction. Each of the reprogramming factors are shown spaced apart with different viral 2A peptides. The inverted repeats (IR) and polyadenylation (pA) sequences are indicated. See “Transposons and induced pluripotent stem cells” for details.
Transposon-based reversible genetic modification as a novel paradigm for iPS induction. Each of the reprogramming factors are shown spaced apart with different viral 2A peptides. The inverted repeats (IR) and polyadenylation (pA) sequences are indicated. See “Transposons and induced pluripotent stem cells” for details.
Conclusions and perspectives
The continued development of improved transposon-based gene delivery approaches is paving the way toward novel clinically relevant gene therapy strategies. The SB and PB transposons currently represent some of the most attractive systems for stable nonviral genetic modification of primary somatic cells and stem or progenitor cells, in particular. The genetic modification of HSCs and T cells with the use of transposons represent a particularly promising avenue. The ability to use SB transposons for stable gene delivery into T cells recently prompted the first-in-man gene therapy trial for lymphoid tumors. Despite these advances, there are still some challenges that would need to be addressed. In particular, robust and safe nonviral transfection technologies enabling efficient uptake of the transposon and transposase constructs into somatic cells would need to be developed further. Furthermore, it will be important to analyze in more detail the possible genotoxic risks of SB and PB transposons with the use of tumor-prone mouse models or in vitro genotoxicity assays, as has been done previously with γ-retroviral or lentiviral vectors.70-74 Moreover, the use of insulator sequences and site-specific targeted integration modalities warrant further optimization and validation with the use of stringent genotoxicity assays. Undoubtedly, lessons learned from previous gene therapy trials with integrating viral vectors are benefiting the implementation of transposon-based gene therapy approaches. To further assess the potential and limitations of transposons for gene therapy, it will be necessary to conduct more exhaustive preclinical efficacy and phenotypic correction in both small and large animal models in anticipation of future gene therapy clinical trials. Finally, thanks to recent insights in transposon biology, it has now become possible to develop a reversible genetic modification paradigm for iPS induction, bringing it one step closer to clinical reality. However, whether iPS cells are truly functionally equivalent to embryonic stem cells is one of the outstanding questions that would still need to be addressed.75 In addition, it would be important to better understand the mechanisms that allow for safe and robust coaxed differentiation of iPS cells while minimizing the risks of inadvertent teratomagenesis. The nonviral iPS cells provides an attractive platform to address this further.
Acknowledgments
We thank all the members of the VIB and MDC teams for their valuable contributions to the various studies that were discussed in this review.
This work was supported by grants from European Union FP5 (JUMPY), FP6 (INTHER), and FP7 (PERSIST), FWO (G.0632.07), KUL GOA/2004/09, NGFN-2.
Authorship
Contribution: All authors (T.V., Z. Ivics, Z. Izsvák, M.K.L.C.) contributed to writing different sections of the review and read, corrected, and edited the manuscript; T.V. is the lead author; and all authors also designed and supervised the experiments that led to some of the breakthroughs highlighted in this manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Thierry VandenDriessche, Flanders Institute for Biotechnology, Vesalius Research Center, University of Leuven, Herestraat 49, Leuven, Belgium; e-mail: thierry.vandendriessche@med.kuleuven.be.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal