Abstract
In this issue of Blood, Tamburini and colleagues have studied the regulation of protein translation control in primary AML cells and describe a mTORC1-independent mechanism of regulation of the translation initiation complex that can be targeted with 4EGI-1, a small molecule inhibitor of translation, leading to death of the cells.1 This and other recent studies add to a growing body of evidence that AML cells have a critical dependence on active protein translation, which may provide an Achilles' heel for the tumor cells that can be targeted therapeutically.
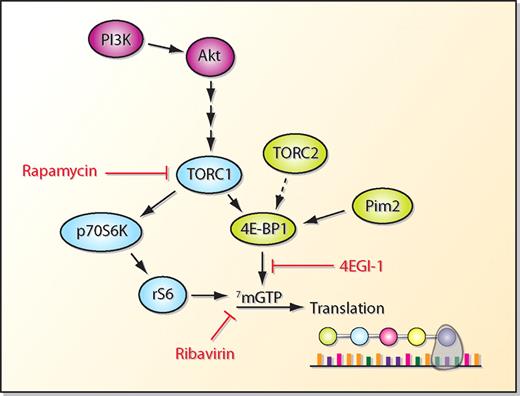
Protein translation in normal hematopoietic cells is regulated by signal transduction pathways that modify 2 proteins, ribosomal S6 (rS6) and eukaryotic translation initiation factor 4E (eIF4E). When activated, both of these proteins bind to the 5′ end of messenger RNA (mRNA) and enhance the binding of mRNA to polysomes, leading to increased translation (see figure). This regulation is particularly important for a subclass of proto-oncogenes that are designated eIF4E-sensitive and include c-myc, cyclin D1, and Bcl-xL.2 Some models of protein translation demonstrate that both of these pathways are coordinately regulated by the mammalian target of rapamycin (mTOR) and in particular by the TORC1 protein complex (mTORC1).3 In acute myeloid leukemia (AML) cells, we and others have demonstrated that the phosphatidylinositol 3-kinase (PI3K) pathway is activated and can regulate phosphorylation of the 70 kDa S6 kinase 1 (S6K1)/rS6 arm of this pathway.4 Rapamycin also inhibits phosphorylation of 4EBP1 but inhibition may not be complete. Rapamycin, the inhibitor of mTORC1, decreases survival of AML cells, particularly after chemotherapy, and has been tested in the therapy of AML. However, activity of single-agent rapamycin derivatives is low, raising the question of whether there are alternative mechanisms regulating protein translation in AML.
Schematic of protein translation regulation. Note that signaling cascades have been simplified for graphic display and that arrows do not always indicate phosphorylation events. For example, phosphorylation of 4E-BP1 leads to its dissociation from mRNA allowing binding of eIF4E to 5′ cap structures. Thus, arrow indicates activation of the pathway, not phosphorylation of target. Similarly, 4EGI-1 is a mimetic of nonphosphorylated 4E-BP1 which inhibits translation by blocking binding of eIF4E to mRNA.
Schematic of protein translation regulation. Note that signaling cascades have been simplified for graphic display and that arrows do not always indicate phosphorylation events. For example, phosphorylation of 4E-BP1 leads to its dissociation from mRNA allowing binding of eIF4E to 5′ cap structures. Thus, arrow indicates activation of the pathway, not phosphorylation of target. Similarly, 4EGI-1 is a mimetic of nonphosphorylated 4E-BP1 which inhibits translation by blocking binding of eIF4E to mRNA.
The role of eIF4E has been independently studied in AML cells, and a recent clinical trial suggests that targeting protein translation may have clinical benefit. eIF4E, particularly when overexpressed, is sufficient to drive protein translation and transform cells. Previous work has demonstrated that, in primary M4 and M5 AML cells, eIF4E is up-regulated compared with bone marrow mononuclear cells, and this up-regulation contributes to leukemogenesis.5 The antiviral compound ribavirin is known to block the ability of eIF4E to bind to mRNA. Based on these data, Assouline and colleagues performed a pilot study of ribavirin in patients with relapsed M4 and M5 AML who were not eligible for chemotherapy. Although the study was small (11 evaluable patients), 1 complete response and 2 partial responses were seen. As with the rapamycin data, the trial suggests that inhibition of protein translation is an interesting target, but our understanding of the process in AML is incomplete.
Tamburini et al have taken on the technically challenging task of addressing these questions using multiple approaches in primary AML cells and AML cell extracts.1 Importantly, they demonstrate that rapamycin, the mTORC1 inhibitor, does not consistently inhibit protein translation in AML cells in culture. Furthermore, rapamycin derivatives failed to inhibit eIF4E assembly or the binding of the c-Myc mRNA to polysomes. Similarly, inhibition of raptor, a critical protein component of mTOR, does not inhibit eIF4E assembly, inhibit binding of mRNA to polysomes, or induce AML cell death. In contrast, the recently described 4E-BP1 mimetic, 4EGI-1, does inhibit protein translation of critical target messages including c-myc and cyclin D1.6 4EGI-1 also consistently induces AML cell death in culture. Finally, Tamburini et al demonstrate that a serine threonine kinase, pim2, which is not regulated by PI3K, can regulate phosphorylation of a critical regulatory site on 4E-BP1. Taken together, these data suggest that there is a critical signaling cascade that regulates protein translation and survival in AML cells through a mTORC1-independent, 4E-BP1/eIF4E–dependent pathway.
These results clarify previous data and raise new, more focused questions. Recent studies have demonstrated that the effect of rapamycin on 4E-BP1 phosphorylation is dependent on the duration of exposure to the drug. It will be important in future studies to determine whether this is true in AML cells as well. Most importantly, it will be essential to determine whether combination therapy with rapamycin and ribavirin or other compounds that inhibit these signaling networks in AML cells will improve therapy in vivo. Tamburini et al have provided a valuable road map for the rational design of such studies.
Conflict-of-interest disclosure: The author receives research support from Sanofi Aventis Corporation. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal