Abstract
The small guanine-nucleotide–binding protein Rap1 plays a key role in platelet aggregation and hemostasis, and we recently identified Rap1GAP2 as the only GTPase-activating protein of Rap1 in platelets. In search of Rap1GAP2-associated proteins, we performed yeast-2-hybrid screening and found synaptotagmin-like protein 1 (Slp1) as a new binding partner. We confirmed the interaction of Rap1GAP2 and Slp1 in transfected COS-1 and HeLa cells and at endogenous level in human platelets. Mapping studies showed that Rap1GAP2 binds through amino acids T524-K525-X-T527 within its C-terminus to the C2A domain of Slp1. Slp1 contains a Rab27-binding domain, and we demonstrate that Rap1GAP2, Slp1, and Rab27 form a trimeric complex in transfected cells and in platelets. Purified Slp1 dose-dependently decreased dense granule secretion in streptolysin-O–permeabilized platelets stimulated with calcium or guanosine 5′-O-[gamma-thio] triphosphate. The isolated C2A domain of Slp1 had a stimulatory effect on granule secretion and reversed the inhibitory effect of full-length Slp1. Purified Rap1GAP2 augmented dense granule secretion of permeabilized platelets, whereas deletion of the Slp1-binding TKXT motif abolished the effect of Rap1GAP2. We conclude that Slp1 inhibits dense granule secretion in platelets and that Rap1GAP2 modulates secretion by binding to Slp1.
Introduction
Blood platelets are essential for hemostasis and play an important role in the development of thrombosis in the vasculature.1 During primary hemostasis, platelets adhere to sites of endothelial damage and the initial platelet coat is soon reinforced by additional platelets forming a stable aggregate. At the same time, platelets secrete their intracellular granules containing substances that further activate platelets in an autocrine loop and affect local coagulation and endothelial and smooth muscle cell functions.2
Two main types of secretory granules have been described in platelets, dense granules, and α-granules. Dense granules contain small molecules, including adenosine diphosphate, adenosine triphosphate, and 5-hydroxytryptamine (5-HT, serotonin), whereas α-granules store many proteins, such as fibrinogen, von Willebrand factor, various cytokines, and growth factors.3,4 Dense granule secretion is regulated by the small GTPase Rab27 that is localized at the granule membrane.5 Rab27 probably mediates its action by promoting granule motility or by enhancing tethering and fusion of granules with the plasma membrane.6,7 For these functions, Rab27 needs to interact with effector proteins, and Munc13-4 was identified as the first Rab27-binding protein in platelets.8 Other proteins involved in dense granule secretion are the small GTPase Ral9 and membrane proteins of the soluble NSF attachment protein receptor family either bound to the granule, such as VAMP-8,10 or to the plasma membrane, such as SNAP-23 and syntaxin 2.11
Granule secretion is accompanied by the development of tight cellular interactions between platelets. This aggregation of platelets is mediated by integrins and other adhesion receptors.2 Integrin activation is tightly regulated by the small guanine-nucleotide-binding protein Rap1, and deletion of the platelet isoform Rap1b in mouse platelets results in a bleeding phenotype.12 Rap1 activity is controlled by GTPase-activating proteins (GAPs) and guanine-nucleotide exchange factors (GEFs). We recently discovered Rap1GAP2 as the only GTPase-activating protein of Rap1 in platelets.13 Rap1GAP2 contains a conserved central catalytic GAP domain, an N-terminal 14-3-3-binding site, and a large C-terminal region of unknown function. Platelet activation results in phosphorylation of Rap1GAP2 on serine 9, binding of 14-3-3 protein, and probably inhibition of GAP function.14 In parallel, activation of platelet-specific GEFs, such as CD-GEF I and III and PDZ-GEF1, results in increased Rap1-GTP levels and thus adhesion and aggregation.13,15
In this article, we demonstrate that the Rab27-binding protein synaptotagmin-like protein 1 (Slp1) is a new direct binding partner of Rap1GAP2 in human platelets. We show that Rap1GAP2 binding to Slp1 is mediated through a short sequence within the C-terminal region of Rap1GAP2; and vice versa, the C2A domain of Slp1 is sufficient for Slp1 binding to Rap1GAP2. Furthermore, we prove the existence of a trimeric complex composed of Rap1GAP2, Slp1, and Rab27 in human platelets, and provide evidence for a regulatory role of Slp1 in platelet dense granule secretion.
Methods
Antibodies, constructs, materials
To detect Rap1GAP2a, a previously described polyclonal antibody was used.13 To detect Slp1, a polyclonal antibody against Slp1 was produced using full-length recombinant glutathione-S-transferase-tagged Slp1 purified from Escherichia coli BL21 as antigen. Immunization of rabbits and subsequent purification were performed by ImmunoGlobe Antikörpertechnik. Commercially available antibodies were used for detection of Rab27a (M02; Abnova), FLAG tag (M2; Sigma-Aldrich), myc tag (A-14, 9E10; Santa Cruz Biotechnology), VSV tag (P5D4; Sigma-Aldrich), and GST tag (GST-2; Sigma-Aldrich). Horseradish peroxidase–coupled goat anti–rabbit and goat anti–mouse were from Dianova and were used as secondary antibodies for immunoblot analysis visualized by the enhanced chemiluminescence method (Millipore, GE Healthcare). Cy3- and Cy5-labeled secondary antibodies were obtained from Jackson ImmunoResearch Laboratories. Rap1GAP2 wild-type peptide with the sequence HNSMEVTKTTFSPPV (amino acids 518-532 of Rap1GAP2a) and Rap1GAP2ΔEVTKTT peptide with the sequence GISHNSMFSPPVVAA (amino acids 515-535 of Rap1GAP2a lacking amino acids 522-527) were obtained from Schafer-N.
Rap1GAP2a13 was FLAG-tagged or hexahistidine-tagged at the C-terminus and expressed using mammalian expression vector pcDNA4/TO (Invitrogen). Site-directed mutagenesis was performed as described.14 Full-length Slp1 cDNA (clone IRATp970G0456D, GenBank accession no. BC035725) was obtained from RZPD and subcloned into EcoRI and XhoI sites of the mammalian expression vector pcDNA3.1/myc-His (Invitrogen). To produce recombinant glutathione-S-transferase fusion protein of Slp1, full-size cDNA was subcloned into pGEX-4T3 vector (GE Healthcare). For hexahistidine-tagged recombinant protein production, cDNA of Slp1 was subcloned into pET28 (Merck). Rab27a-containing plasmid pEGFP-C3, a kind gift from M. C. Seabra (Imperial College London, London, United Kingdom), was used to subclone Rab27a VSV-tagged at the C-terminus into HindIII and XhoI sites of pcDNA4/TO (Invitrogen). Hexahistidine-tagged Rap1b cloned in pET28 and glutathione-S-transferase-tagged 14-3-3β cloned in pGEX-4T3 were described before.14 All constructs were sequence-verified.
Unless otherwise specified, all chemicals were obtained from Sigma-Aldrich, except for streptolysin-O, which was kindly provided by S. Bhakdi (Johannes Gutenberg University, Mainz, Germany).
Protein purification
GST-Slp1, GST-C2A, GST-14-3-3β, and His6-Slp1 were expressed in E coli BL21 and affinity purified using glutathione-Sepharose 4B beads (GE Healthcare) or nickel-nitrolotriacetic acid-agarose (QIAGEN), respectively. His6-tagged recombinant Rap1GAP2 wild-type and Rap1GAP2Δ522-527 mutant were affinity purified from COS-1 cells. Briefly, 100 10-cm dishes of COS-1 were transiently transfected with the appropriate plasmids. At 48 hours after transfection, cells were lysed with lysis buffer (50 mM NaH2PO4/NaOH, pH 8, 300 mM NaCl, 1% [wt/vol] Triton-X 100) containing protease inhibitors, and His6-tagged proteins were affinity purified using Ni-NTA agarose (QIAGEN). His6-Rap1b was purified from E coli BL21 via HisTrap HP column (GE Healthcare) using an ÄKTA-system and subsequently loaded with GTP as described before.14 The purity of all proteins was examined by sodium dodecyl sulfate–polyacrylamide gel electrophoresis followed by Coomassie blue staining.
Yeast-2-hybrid screening
The MATCHMAKER 2-Hybrid System 3 (Clontech-Takara Bio) was used with full-length Rap1GAP2a as bait and human adult brain cDNA library as prey as described.14
Cell preparation, transfection, lysis, immunoprecipitation, and pull-down experiments
Culture and transfection of COS-1 and HeLa cells have been described previously.14 Venous blood was drawn from healthy volunteers taking no medications who gave their informed consent according to the Declaration of Helsinki. Washed platelets were obtained by sequential centrifugation as described.16 Cell lysis, immunoprecipitation, and pull-down assays were performed as described14 using 5 μL ANTI-FLAG M2 Affinity Gel (Sigma-Aldrich) or 10 μg anti-Rab27a antibody (Abnova) or 1 μL or 5 μL glutathione-Sepharose 4B suspension (GE Healthcare) saturated with GST, GST-Slp1, or GST-14-3-3β, respectively. Sampling of human blood for the isolation of platelets was performed in accordance with the Review Board of Frankfurt University Medical School and the Human Research Ethics Committee of University College Dublin.
Peptide binding assay (PepSpot)
Synthetic peptides with either wild-type Rap1GAP2a sequence HNSMEVTKTTFSPPV or with one amino acid mutated to alanine or key threonines phosphorylated were synthesized on cellulose membrane (ImmunoGlobe Antikörpertechnik) and incubated with 1 μg/mL purified recombinant GST-Slp1 in Tris-buffered saline with 0.1% Tween 20 and 5% bovine serum albumin (BSA) for 3 hours at room temperature. Bound GST-Slp1 was visualized by immunoblotting using a monoclonal anti-GST antibody (Sigma-Aldrich).
Confocal microscopy
HeLa cells were grown on glass coverslips and transfected with expression vectors for EGFP-tagged Rab27a, VSV-tagged Rap1GAP2, and myc-tagged Slp1. At 24 hours after transfection, cells were fixed with 3.7% paraformaldehyde in phosphate-buffered saline (PBS) for 15 minutes on ice, washed with PBS, and then permeabilized with 0.2% Triton X-100 in PBS for 10 minutes at room temperature. To detect VSV-tagged Rap1GAP2 and myc-tagged Slp1, primary tag-specific antibodies diluted in PBS with 1% BSA were added and incubated for 1 hour at 37°C followed by incubation with Cy5-conjugated anti–mouse IgG and Cy3-conjugated anti–rabbit IgG as secondary antibodies. After further washing with PBS and water, samples were mounted in GEL/Mount (Biomeda). Staining was observed using a Zeiss LSM 510 confocal laser scanning microscope equipped with a Plan-Apochromat 63×/1.4 oil DIC objective lens and LSM 510 META software (Carl Zeiss).
Assay for secretion of platelet dense granules
Freshly obtained washed platelets (∼108 platelets/mL, counted with CASY Cell Counter, Innovatis AG) were resuspended in 70 μL prewarmed buffer A (50 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid/KOH, pH 7.4, 78 mM KCl, 4 mM MgCl2, 2 mM ethyleneglycoltetraacetic acid, 0.2 mM CaCl2, 5 mM dithiothreitol) containing 4 mg/mL BSA, 5 mM adenosine triphosphate (Roche), 8 mM creatine phosphate (Fluka), and 50 μg/mL creatine phosphokinase (Sigma-Aldrich). Then, platelets were permeabilized using 0.6 μg/mL streptolysin-O in buffer A containing 4 mg/mL BSA at 30°C for 5 minutes. Permeabilized platelets were placed on ice and incubated with purified proteins or peptides to be tested for 40 minutes followed by further incubation at 30°C for 5 minutes. Finally, platelets were stimulated with either 10 μL prewarmed buffer A, where the free calcium ion concentration was calculated to approximately 20 nM, or 10 μL prewarmed stimulation buffer (50 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid/KOH, pH 7.4, 78 mM KCl, 4 mM MgCl2, 2 mM ethyleneglycoltetraacetic acid, 20 mM CaCl2), which results in 20 μM free calcium, at 30°C for 1 minute. In case of guanosine 5′-O-[gamma-thio]triphosphate (GTP-γS)-induced secretion of dense granules, platelets were stimulated with 100 μM GTP-γS at 30°C for 5 minutes. The reaction was stopped by addition of 200 μL 2-fold concentrated ice-cold stop buffer (100 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid/KOH, pH 7.4, 156 mM KCl, 8 mM MgCl2, 18 mM ethyleneglycoltetraacetic acid, 0.4 mM CaCl2) and incubation on ice for 5 minutes. Then, platelets were removed by centrifugation at 4°C 5000g for 5 minutes, and released serotonin (5-HT) in the supernatant was measured using Walac Victor 1420 Multilabel Counter as described.17 The secretion levels of serotonin were expressed as percentage of total serotonin of permeabilized platelets before the final centrifugation. Data are the mean plus or minus SEM of at least 3 independent experiments performed in triplicate. The statistical significance of the means was analyzed by analysis of variance and Bonferroni posttest (95% confidence interval) using GraphPad Prism software, Version 4.0. P values below .05 are considered statistically significant. Protein concentrations were determined by the Bradford method (Bio-Rad) and from intensities of the bands in Coomassie blue–stained sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels using BSA as standard. Permeabilization was monitored by immunoblotting using anti-LDH antibody (Chemicon International).
In vitro GAP assay
In vitro GAP assay was performed as described previously.14
Results
Rap1GAP2 interacts with Slp1
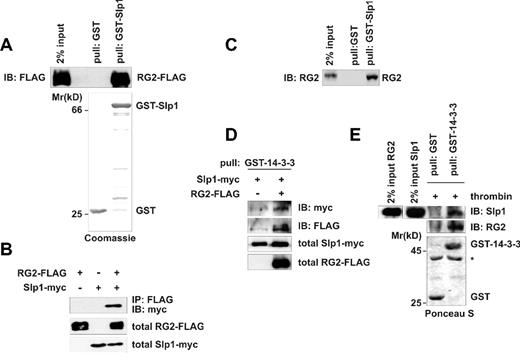
We were interested in identifying proteins that might be involved in the regulation of Rap1GAP2.13 For this purpose, we performed yeast-2-hybrid screening using full-length wild-type Rap1GAP2a, the predominant splice isoform of Rap1GAP2 in platelets, as bait and human adult brain cDNA library as prey. Among the library clones that were found to interact strongly, 2 clones were identified as synaptotagmin-like protein 1 (Slp1, also called JFC1).18,19 These clones contained the C-terminal part of Slp1 composing 2 tandem C2 domains, C2A and C2B. To confirm direct binding of Slp1 to Rap1GAP2, we performed pull-down assays using COS-1 cell lysates overexpressing FLAG-tagged Rap1GAP2 and purified recombinant full-length Slp1 as GST fusion protein. GST-Slp1 was clearly able to pull down transfected Rap1GAP2 from cell lysates, whereas GST alone did not bind Rap1GAP2 (Figure 1A). To verify that Rap1GAP2 and Slp1 interact also in intact mammalian cells, we performed coimmunoprecipitation experiments. COS-1 cells were transfected with epitope-tagged versions of Rap1GAP2 and Slp1 either alone or in combination, and Rap1GAP2 was immunoprecipitated with anti-FLAG antibody. Analysis of the precipitates by immunoblotting with anti-myc antibody revealed the presence of Slp1 only in precipitates from cells overexpressing both Slp1 and Rap1GAP2 (Figure 1B lane 3). To assess the Rap1GAP2/Slp1 interaction in a more physiologic context, we performed pull-down assays using human platelet lysates containing endogenous Rap1GAP2 protein. Only GST-Slp1, but not GST alone, bound to endogenous Rap1GAP2 (Figure 1C). To determine whether Slp1 is expressed in human platelets, we generated an antibody against full-length human Slp1. The antibody specifically recognized the 66-kDa Slp1 protein in HeLa cells transfected with Slp1 but not in mock-transfected cells (data not shown). Importantly, the antibody recognized a band of similar molecular weight in human platelet lysate, suggesting that Slp1 is endogenously expressed in human platelets. Neither our Rap1GAP2 antibody nor the newly generated anti-Slp1 antibody was able to immunoprecipitate their antigens efficiently from human platelet lysates. To obtain conclusive evidence that endogenous Rap1GAP2 and Slp1 interact, we applied an alternative precipitation approach as previously described.14 We exploited the ability of 14-3-3 proteins to bind to Rap1GAP2 at phosphorylated serine 9 within the N-terminus of Rap1GAP2; 14-3-3 precipitates Rap1GAP2 from platelet lysate, and thrombin treatment was shown to enhance this interaction.14 We also demonstrated that 14-3-3 and Slp1 do not interact directly with each other (Slp1 is designated Rip2 in Figure 3D).14 To further exclude a possible direct interaction of Slp1 and 14-3-3, we performed an additional experiment using purified recombinant GST-14-3-3. In lysates of transfected cells, 14-3-3 was able to bind to Slp1 only in the presence of Rap1GAP2 (Figure 1D). Finally, we used purified recombinant GST-14-3-3 to precipitate Rap1GAP2 from lysates of thrombin-treated platelets and were able to coprecipitate endogenous Slp1 (Figure 1E lane 4). A control experiment showed that GST alone did not bind Rap1GAP2 or Slp1 (Figure 1E lane 3). Taken together, these experiments confirm that the interaction between Rap1GAP2 and Slp1 is direct and that both proteins interact in transfected cells as well as at endogenous level in human platelets.
Rap1GAP2 interacts with Slp1. (A) Pull-down of transfected Rap1GAP2 with GST-Slp1. COS-1 cells were transfected with FLAG-tagged Rap1GAP2 (RG2-FLAG). (Bottom panel) Equal amounts of GST as control and GST-Slp1 coupled to GSH-Sepharose beads were used for precipitation. (Top panel) Bound Rap1GAP2 protein was visualized by immunoblot using anti-FLAG antibody. Expression level of Rap1GAP2 is shown as 2% input of total RG2-FLAG. The broad band of Rap1GAP2 is probably the result of extensive posttranslational modifications. (B) Coimmunoprecipitation of transfected Rap1GAP2 and Slp1. COS-1 cells were transfected with FLAG-tagged Rap1GAP2, myc-tagged Slp1, or FLAG-tagged Rap1GAP2 together with myc-tagged Slp1. After lysis, Rap1GAP2 was precipitated with anti-FLAG antibody. The precipitates were analyzed for the presence of bound Slp1 by immunoblot using anti-myc antibody. (Top panel) Precipitation results. (Bottom 2 panels) Transfection levels of Rap1GAP2 (total RG2-FLAG, 2% input) and Slp1 (total Slp1-myc, 2% input). (C) Pull-down of endogenous Rap1GAP2 with GST-Slp1 from human platelets. Equal amounts of GST as control and GST-Slp1 coupled to GSH-Sepharose beads were incubated with human platelet lysate. Bound endogenous Rap1GAP2 protein was visualized with anti-Rap1GAP2 antibody (RG2). Expression level of Rap1GAP2 is shown as 2% input of total RG2. (D) Pull-down of transfected Rap1GAP2 and Slp1 with GST-14-3-3. COS-1 cells were transfected with myc-tagged Slp1 and without or with FLAG-tagged Rap1GAP2 (RG2-FLAG). Cells were lysed, and GST-14-3-3β was used to pull down Rap1GAP2 and indirectly Slp1 bound to Rap1GAP2. (Top panel) Precipitated Slp1 was visualized by immunoblotting using anti-myc antibody. (Second panel from top) Precipitation of Rap1GAP2 was controlled by immunoblot with anti-FLAG antibody. In parallel, total cell lysates were analyzed for the expression of Slp1 (total Slp1-myc, 2% input) and Rap1GAP2 (total RG2-FLAG, 2% input). (E) Pull-down of endogenous Rap1GAP2 and Slp1 from human platelets. Lysates from thrombin-treated platelets were subjected to pull-down assays using either GST as control or GST-14-3-3β. (First and second panels from top) The precipitates were analyzed for the presence of endogenous Rap1GAP2 (RG2) and Slp1 using specific anti-Rap1GAP2 and anti-Slp1 antibodies. Expression levels of Rap1GAP2 and Slp1 are shown as 2% input of total protein amounts. (Bottom panel) The amounts of GST and GST-14-3-3β used for precipitation. *Unspecific band.
Rap1GAP2 interacts with Slp1. (A) Pull-down of transfected Rap1GAP2 with GST-Slp1. COS-1 cells were transfected with FLAG-tagged Rap1GAP2 (RG2-FLAG). (Bottom panel) Equal amounts of GST as control and GST-Slp1 coupled to GSH-Sepharose beads were used for precipitation. (Top panel) Bound Rap1GAP2 protein was visualized by immunoblot using anti-FLAG antibody. Expression level of Rap1GAP2 is shown as 2% input of total RG2-FLAG. The broad band of Rap1GAP2 is probably the result of extensive posttranslational modifications. (B) Coimmunoprecipitation of transfected Rap1GAP2 and Slp1. COS-1 cells were transfected with FLAG-tagged Rap1GAP2, myc-tagged Slp1, or FLAG-tagged Rap1GAP2 together with myc-tagged Slp1. After lysis, Rap1GAP2 was precipitated with anti-FLAG antibody. The precipitates were analyzed for the presence of bound Slp1 by immunoblot using anti-myc antibody. (Top panel) Precipitation results. (Bottom 2 panels) Transfection levels of Rap1GAP2 (total RG2-FLAG, 2% input) and Slp1 (total Slp1-myc, 2% input). (C) Pull-down of endogenous Rap1GAP2 with GST-Slp1 from human platelets. Equal amounts of GST as control and GST-Slp1 coupled to GSH-Sepharose beads were incubated with human platelet lysate. Bound endogenous Rap1GAP2 protein was visualized with anti-Rap1GAP2 antibody (RG2). Expression level of Rap1GAP2 is shown as 2% input of total RG2. (D) Pull-down of transfected Rap1GAP2 and Slp1 with GST-14-3-3. COS-1 cells were transfected with myc-tagged Slp1 and without or with FLAG-tagged Rap1GAP2 (RG2-FLAG). Cells were lysed, and GST-14-3-3β was used to pull down Rap1GAP2 and indirectly Slp1 bound to Rap1GAP2. (Top panel) Precipitated Slp1 was visualized by immunoblotting using anti-myc antibody. (Second panel from top) Precipitation of Rap1GAP2 was controlled by immunoblot with anti-FLAG antibody. In parallel, total cell lysates were analyzed for the expression of Slp1 (total Slp1-myc, 2% input) and Rap1GAP2 (total RG2-FLAG, 2% input). (E) Pull-down of endogenous Rap1GAP2 and Slp1 from human platelets. Lysates from thrombin-treated platelets were subjected to pull-down assays using either GST as control or GST-14-3-3β. (First and second panels from top) The precipitates were analyzed for the presence of endogenous Rap1GAP2 (RG2) and Slp1 using specific anti-Rap1GAP2 and anti-Slp1 antibodies. Expression levels of Rap1GAP2 and Slp1 are shown as 2% input of total protein amounts. (Bottom panel) The amounts of GST and GST-14-3-3β used for precipitation. *Unspecific band.
C2A domain of Slp1 is sufficient for binding of Slp1 to Rap1GAP2
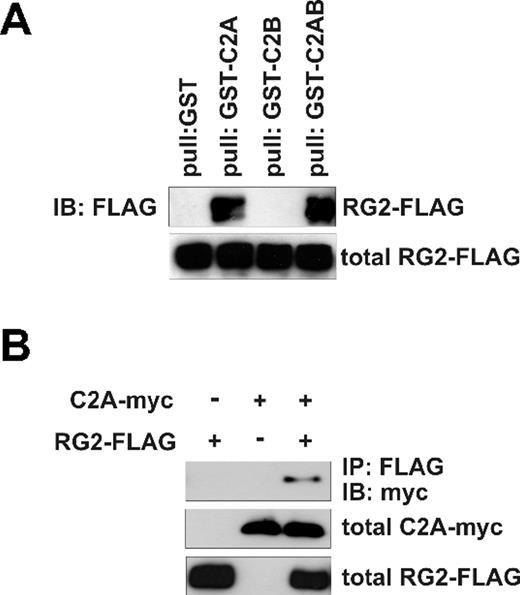
Slp1 is composed of an N-terminal Rab27-binding site and 2 C2 domains, C2A and C2B at the C-terminus.18,19 To determine the Rap1GAP2-binding site within Slp1, we generated Slp1 mutants composed of C2A, C2B, or C2AB domain fused to GST. From our yeast-2-hybrid result, we assumed the binding site for Rap1GAP2 to be located within the 2 C2 domains of Slp1. As shown in Figure 2A, only GST-C2A and GST-C2AB were able to pull down transfected Rap1GAP2 from HeLa cell lysates, indicating that the C2A domain of Slp1 is required and sufficient for binding of Slp1 to Rap1GAP2. This result was further confirmed in intact mammalian cells. In immunoprecipitation experiments using HeLa cell lysates overexpressing FLAG-tagged Rap1GAP2 and myc-tagged C2A, we were able to coimmunoprecipitate C2A together with Rap1GAP2 (Figure 2B lane 3). Binding activities of some C2 domains are modulated by calcium ions20 ; however, the Rap1GAP2/Slp1 interaction was not affected by calcium depletion in pull-down assays (data not shown).
C2A domain of Slp1 is sufficient for binding of Slp1 to Rap1GAP2. (A) Pull-down of transfected Rap1GAP2 with GST-Slp1 mutants. Lysates of HeLa cells overexpressing FLAG-tagged Rap1GAP2 (RG2-FLAG) were subjected to pull-down experiments using equal amounts of GST as control and GST fusion proteins of C2A (amino acids 292-393 of Slp1), C2B (amino acids 433-580 of Slp1), and C2AB (amino acids 292-580 of Slp1). (Top panel) The precipitates were analyzed for the presence of bound Rap1GAP2 by immunoblot with anti-FLAG antibody. (Bottom panel) Expression levels of Rap1GAP2 (total RG2-FLAG, 2% input). (B) Coimmunoprecipitation of transfected Rap1GAP2 and Slp1-C2A. HeLa cells were transfected with FLAG-tagged Rap1GAP2, myc-tagged C2A domain of Slp1, or FLAG-tagged Rap1GAP2 together with myc-tagged Slp1-C2A. After lysis, Rap1GAP2 was precipitated with anti-FLAG antibody. (Top panel) The precipitates were analyzed for the presence of bound Slp1-C2A by immunoblot using anti-myc antibody. (Bottom 2 panels) Expression levels of Slp1-C2A (total C2A-myc, 2% input) and Rap1GAP2 (total RG2-FLAG, 2% input).
C2A domain of Slp1 is sufficient for binding of Slp1 to Rap1GAP2. (A) Pull-down of transfected Rap1GAP2 with GST-Slp1 mutants. Lysates of HeLa cells overexpressing FLAG-tagged Rap1GAP2 (RG2-FLAG) were subjected to pull-down experiments using equal amounts of GST as control and GST fusion proteins of C2A (amino acids 292-393 of Slp1), C2B (amino acids 433-580 of Slp1), and C2AB (amino acids 292-580 of Slp1). (Top panel) The precipitates were analyzed for the presence of bound Rap1GAP2 by immunoblot with anti-FLAG antibody. (Bottom panel) Expression levels of Rap1GAP2 (total RG2-FLAG, 2% input). (B) Coimmunoprecipitation of transfected Rap1GAP2 and Slp1-C2A. HeLa cells were transfected with FLAG-tagged Rap1GAP2, myc-tagged C2A domain of Slp1, or FLAG-tagged Rap1GAP2 together with myc-tagged Slp1-C2A. After lysis, Rap1GAP2 was precipitated with anti-FLAG antibody. (Top panel) The precipitates were analyzed for the presence of bound Slp1-C2A by immunoblot using anti-myc antibody. (Bottom 2 panels) Expression levels of Slp1-C2A (total C2A-myc, 2% input) and Rap1GAP2 (total RG2-FLAG, 2% input).
Rap1GAP2/Slp1 interaction is mediated through the TKXT motif within the C-terminus of Rap1GAP2
To map the binding site for Slp1 in Rap1GAP2, we generated truncation mutants of Rap1GAP2 and performed GST-Slp1 pull-down experiments. As shown in Figure 3A, Slp1 bound to wild-type Rap1GAP2 and Rap1GAP2ΔNterm mutant lacking the N-terminal part with equal potency, whereas no binding could be detected between Slp1 and a C-terminally truncated Rap1GAP2 mutant (Rap1GAP2ΔCterm). By further truncational analysis (data not shown), we narrowed down the interaction site to a short sequence of 6 residues in the C-terminal region of Rap1GAP2. To confirm the role of these residues for Slp1 binding, we generated a Rap1GAP2 deletion mutant lacking the suspected sequence from position 522 to 527 and a control mutant, where an arbitrarily chosen sequence in proximity was deleted. We tested both mutants in GST-Slp1 pull-down experiments. The control mutant bound Slp1 comparable with wild-type Rap1GAP2 (Figure 3B lanes 1 and 2), whereas Rap1GAP2Δ522-527 mutant did not bind Slp1 (Figure 3B lane 3). From this result, we conclude that the EVTKTT sequence (amino acids 522-527 of Rap1GAP2) is required for binding of Slp1 to Rap1GAP2. To determine the role of each amino acid residue of this motif, we generated consecutive alanine point mutants of the EVTKTT sequence and performed GST-Slp1 pull-down assays (Figure 3C). We observed that mutation of E522 to alanine did not affect binding of Slp1 to Rap1GAP2 (Figure 3C lanes 2 and 3), mutation of V523 or T526 to alanine only slightly reduced binding (Figure 3C lanes 4 and 7), whereas mutation of T524, K525, or T527 almost completely abolished binding of Slp1 to Rap1GAP2 (Figure 3C lanes 5, 6, and 8). These results indicate that amino acids T524, K525, and T527, the so-called TKXT motif, are important for binding of Slp1 to Rap1GAP2. To confirm these data using a complementary approach, we performed peptide-binding assays. For this purpose, short peptides containing the key sequence EVTKTT in wild-type form or with one amino acid mutated to alanine were synthesized on a cellulose membrane and incubated with purified GST-Slp1. As shown in Figure 3D, if T524, K525, and T527 were mutated, no binding of GST-Slp1 could be detected. Interestingly, mutation of E522 to alanine led to a stronger binding of GST-Slp1. We conclude that the residues T524, K525, and T527 in the C-terminal part of Rap1GAP2 constitute the binding site for Slp1. To answer the question whether Slp1 binding to Rap1GAP2 would involve phosphoryation of the threonine residues in the TKXT motif, we tested peptides having the key threonines T524, T527, or both phosphorylated. No binding of GST-Slp1 to the phosphorylated versions of the TKXT motif could be observed (Figure 3D lanes 9-11), indicating that phosphorylation of the TKXT motif of Rap1GAP2 is not required and could even abolish Slp1 binding. Finally, we wanted to test whether peptides containing the key sequence EVTKTT or peptides carrying a deletion of the EVTKTT motif could affect binding of Slp1 to Rap1GAP2. In a pull-down assay using GST-Slp1, addition of the wild-type peptide to platelet lysate blocked the interaction of Slp1 and Rap1GAP2, whereas addition of the mutant peptide did not affect binding of Slp1 to Rap1GAP2 (Figure 3E).
Binding of Rap1GAP2 to Slp1 is mediated through the TKXT motif within the C-terminus of Rap1GAP2. (A) Pull-down of transfected Rap1GAP2 truncation mutants with GST-Slp1. HeLa cells were transfected with either FLAG-tagged Rap1GAP2 (RG2-FLAG) wild-type or different Rap1GAP2 truncation mutants. Rap1GAP2ΔCterm lacks amino acids 467-715, whereas Rap1GAP2ΔNterm lacks amino acids 1-121. Cells were lysed, and pull-down assays using GST or GST-Slp1 were performed. Precipitates were analyzed for the presence of FLAG-tag containing proteins by immunoblot with anti-FLAG antibody. (Top panel) Precipitation results. (Bottom panel) Expression levels of RG2 proteins (total RG2-FLAG, 2% input). (B) Pull-down of transfected Rap1GAP2 deletion mutants with GST-Slp1. Lysates of HeLa cells overexpressing either FLAG-tagged Rap1GAP2 wild-type (RG2-FLAGwt) or deletion mutants Rap1GAP2Δ536-542 as control and Rap1GAP2Δ522-527 were subjected to GST-Slp1 pull-down assays followed by immunoblot analysis using anti-FLAG antibody. (Top panel) Precipitation results. (Bottom panel) Expression levels of RG2 proteins (total RG2-FLAG, 2% input). (C) Pull-down of transfected Rap1GAP2 alanine point mutants with GST-Slp1. HeLa cells were transfected with FLAG-tagged Rap1GAP2 wild-type (RG2-FLAGwt) or different Rap1GAP2 point mutants having each of the amino acids within the EVTKTT sequence (amino acids 522-527) changed to alanine as indicated. Cells were lysed, and lysates were subjected to GST-Slp1 pull-down assays followed by immunoblot analysis with anti-FLAG antibody. (Top panel) Precipitation results. (Bottom panel) Expression levels of RG2 proteins (total RG2-FLAG, 2% input). (D) Peptide binding assay (PepSpot). Synthetic Rap1GAP2 (RG2) peptides covalently bound to cellulose membrane containing either wild-type EVTKTT sequence of Rap1GAP2 or with consecutive amino acid changed to alanine (A) or phosphorylated threonine residues (pT) as indicated were subjected to GST-Slp1 overlay assay followed by immunoblot analysis using anti-GST antibody. (E) Pull-down of endogenous Rap1GAP2 with GST-Slp1 from human platelet lysates in absence or presence of Rap1GAP2 peptides. Human platelet lysate or lysate supplemented with Rap1GAP2 wild-type (RG2wt) peptide (amino acids 518-532 of Rap1GAP2) or Rap1GAP2ΔEVTKTT (RG2ΔEVTKTT) peptide (amino acids 515-535 of Rap1GAP2 lacking amino acids 522-527) in dimethyl sulfoxide 100 μM each were subjected to GST-Slp1 pull-down assays. The presence of endogenous Rap1GAP2 protein (RG2) was analyzed by immunoblot using anti-Rap1GAP2 antibody. (Top panel) Precipitation results. (Bottom panel) Expression levels of endogenous Rap1GAP2 protein (total RG2, 2% input).
Binding of Rap1GAP2 to Slp1 is mediated through the TKXT motif within the C-terminus of Rap1GAP2. (A) Pull-down of transfected Rap1GAP2 truncation mutants with GST-Slp1. HeLa cells were transfected with either FLAG-tagged Rap1GAP2 (RG2-FLAG) wild-type or different Rap1GAP2 truncation mutants. Rap1GAP2ΔCterm lacks amino acids 467-715, whereas Rap1GAP2ΔNterm lacks amino acids 1-121. Cells were lysed, and pull-down assays using GST or GST-Slp1 were performed. Precipitates were analyzed for the presence of FLAG-tag containing proteins by immunoblot with anti-FLAG antibody. (Top panel) Precipitation results. (Bottom panel) Expression levels of RG2 proteins (total RG2-FLAG, 2% input). (B) Pull-down of transfected Rap1GAP2 deletion mutants with GST-Slp1. Lysates of HeLa cells overexpressing either FLAG-tagged Rap1GAP2 wild-type (RG2-FLAGwt) or deletion mutants Rap1GAP2Δ536-542 as control and Rap1GAP2Δ522-527 were subjected to GST-Slp1 pull-down assays followed by immunoblot analysis using anti-FLAG antibody. (Top panel) Precipitation results. (Bottom panel) Expression levels of RG2 proteins (total RG2-FLAG, 2% input). (C) Pull-down of transfected Rap1GAP2 alanine point mutants with GST-Slp1. HeLa cells were transfected with FLAG-tagged Rap1GAP2 wild-type (RG2-FLAGwt) or different Rap1GAP2 point mutants having each of the amino acids within the EVTKTT sequence (amino acids 522-527) changed to alanine as indicated. Cells were lysed, and lysates were subjected to GST-Slp1 pull-down assays followed by immunoblot analysis with anti-FLAG antibody. (Top panel) Precipitation results. (Bottom panel) Expression levels of RG2 proteins (total RG2-FLAG, 2% input). (D) Peptide binding assay (PepSpot). Synthetic Rap1GAP2 (RG2) peptides covalently bound to cellulose membrane containing either wild-type EVTKTT sequence of Rap1GAP2 or with consecutive amino acid changed to alanine (A) or phosphorylated threonine residues (pT) as indicated were subjected to GST-Slp1 overlay assay followed by immunoblot analysis using anti-GST antibody. (E) Pull-down of endogenous Rap1GAP2 with GST-Slp1 from human platelet lysates in absence or presence of Rap1GAP2 peptides. Human platelet lysate or lysate supplemented with Rap1GAP2 wild-type (RG2wt) peptide (amino acids 518-532 of Rap1GAP2) or Rap1GAP2ΔEVTKTT (RG2ΔEVTKTT) peptide (amino acids 515-535 of Rap1GAP2 lacking amino acids 522-527) in dimethyl sulfoxide 100 μM each were subjected to GST-Slp1 pull-down assays. The presence of endogenous Rap1GAP2 protein (RG2) was analyzed by immunoblot using anti-Rap1GAP2 antibody. (Top panel) Precipitation results. (Bottom panel) Expression levels of endogenous Rap1GAP2 protein (total RG2, 2% input).
Rap1GAP2, Slp1, and Rab27 form a trimeric complex
Slp1 has been shown to bind to Rab27, a small GTPase involved in vesicle-regulated exocytosis of many cell types.21 Rab27 is expressed in 2 isoforms (Rab27a and Rab27b) that share approximately 71% identity at amino acid level. In platelets, both Rab27 isoforms are present.22 To demonstrate that Slp1 interacts with endogenous Rab27, we performed GST-Slp1 pull-down experiments using platelet lysates (Figure 4A). The used monoclonal anti-Rab27a antibody recognized both Rab27 isoforms (data not shown) so that the band for Rab27 probably represents a mixture of Rab27a and Rab27b. In reverse experiments using purified recombinant GST-Rab27a and GST-Rab27b, binding of endogenous Slp1 from human platelet lysates could be observed to both isoforms of Rab27; in addition, binding of Slp1 to Rab27 was independent of the nucleotide state of the Rab protein (data not shown). To determine whether Slp1, Rab27, and Rap1GAP2 form a trimeric complex in vivo, we transfected HeLa cells with epitope-tagged Slp1, Rab27a, and Rap1GAP2 and performed coimmunoprecipitation experiments using anti-FLAG antibody. Rab27a could be detected only in precipitates from cell lysates overexpressing all 3 proteins, indicating that Rab27a, Slp1, and Rap1GAP2 indeed form a complex in intact mammalian cells (Figure 4B). This result could be confirmed in a reverse experiment using anti-VSV antibody (data not shown). We were also able to coimmunoprecipitate endogenous Rab27, Slp1, and Rap1GAP2 from human platelet lysates using anti-Rab27 antibody (Figure 4C). To investigate the subcellular localization of Rab27, Slp1, and Rap1GAP2, HeLa cells were cotransfected with EGFP-tagged Rab27a, myc-tagged Slp1, and VSV-tagged Rap1GAP2. The cells were fixed, permeabilized, and immunostained with tag-specific primary and dye-labeled secondary antibodies. Immunofluorescence analysis revealed a partial colocalization of all 3 proteins in the cytosol as well as at the plasma membrane (Figure 4D). Altogether, these data provide strong evidence for the existence of a trimeric complex composed of Slp1, Rab27, and Rap1GAP2 in transfected cells and in platelets.
Rap1GAP2, Slp1, and Rab27 form a trimeric complex in vivo. (A) Pull-down of endogenous Rab27 with GST-Slp1 from human platelets. Human platelet lysate was subjected to GST-Slp1 pull-down assay followed by immunoblot analysis using anti-Rab27 antibody, which recognizes both isoforms, Rab27a and Rab27b. (Top panel) Precipitation results. (Bottom panel) Expression levels of endogenous Rab27 protein (total Rab27, 2% input). (B) Coimmunoprecipitation of transfected Rap1GAP2 in complex with Slp1 and Rab27a. HeLa cells were transfected either with VSV-tagged Rab27a alone, together with FLAG-tagged Rap1GAP2, or with FLAG-tagged Rap1GAP2 and myc-tagged Slp1. At 24 hours after transfection, cells were lysed, and Rap1GAP2 was immunoprecipitated using anti-FLAG antibody. The precipitates were analyzed for the presence of Rab27a using anti-VSV antibody. (Top panel) Precipitation results. *Immunoglobulin heavy and light chains. (Bottom 3 panels) Expression levels of transfected Rab27a-VSV, Slp1-myc, and Rap1GAP2-FLAG, each as 2% input. (C) Coimmunoprecipitation of endogenous Rap1GAP2 in complex with Slp1 and Rab27 from human platelets. Human platelet lysate containing endogenous Rab27, Rap1GAP2, and Slp1 was subjected to coimmunoprecipitation using anti-Rab27a antibody. (First and second panels from top) The precipitates were examined for the presence of Rap1GAP2 and Slp1 by immunoblot using specific anti-Rap1GAP2 and anti-Slp1 antibodies. (Third panel from top) Amounts of precipitated Rab27 were controlled with anti-Rab27a antibody. As indicated in panel A, the Rab27 band probably consists of both isoforms, Rab27a and Rab27b. Arrowhead represents precipitated Rab27. *Immunoglobulin light chain. (Bottom 3 panels) Expression levels of endogenous Rap1GAP2 (total RG2), Slp1 (total Slp1), and Rab27 (total Rab27), each as 2% input. Vertical lines have been inserted to indicate a repositioned gel lane in the first and second panels from top as well as in the bottom panel. (D) Colocalization of transfected Rap1GAP2, Slp1, and Rab27a. Colocalization of EGFP-tagged Rab27a, VSV-tagged Rap1GAP2, and myc-tagged Slp1 overexpressed in HeLa cells was analyzed by immunofluorescence as described in “Confocal microscopy.” Arrows represent colocalization of all 3 proteins.
Rap1GAP2, Slp1, and Rab27 form a trimeric complex in vivo. (A) Pull-down of endogenous Rab27 with GST-Slp1 from human platelets. Human platelet lysate was subjected to GST-Slp1 pull-down assay followed by immunoblot analysis using anti-Rab27 antibody, which recognizes both isoforms, Rab27a and Rab27b. (Top panel) Precipitation results. (Bottom panel) Expression levels of endogenous Rab27 protein (total Rab27, 2% input). (B) Coimmunoprecipitation of transfected Rap1GAP2 in complex with Slp1 and Rab27a. HeLa cells were transfected either with VSV-tagged Rab27a alone, together with FLAG-tagged Rap1GAP2, or with FLAG-tagged Rap1GAP2 and myc-tagged Slp1. At 24 hours after transfection, cells were lysed, and Rap1GAP2 was immunoprecipitated using anti-FLAG antibody. The precipitates were analyzed for the presence of Rab27a using anti-VSV antibody. (Top panel) Precipitation results. *Immunoglobulin heavy and light chains. (Bottom 3 panels) Expression levels of transfected Rab27a-VSV, Slp1-myc, and Rap1GAP2-FLAG, each as 2% input. (C) Coimmunoprecipitation of endogenous Rap1GAP2 in complex with Slp1 and Rab27 from human platelets. Human platelet lysate containing endogenous Rab27, Rap1GAP2, and Slp1 was subjected to coimmunoprecipitation using anti-Rab27a antibody. (First and second panels from top) The precipitates were examined for the presence of Rap1GAP2 and Slp1 by immunoblot using specific anti-Rap1GAP2 and anti-Slp1 antibodies. (Third panel from top) Amounts of precipitated Rab27 were controlled with anti-Rab27a antibody. As indicated in panel A, the Rab27 band probably consists of both isoforms, Rab27a and Rab27b. Arrowhead represents precipitated Rab27. *Immunoglobulin light chain. (Bottom 3 panels) Expression levels of endogenous Rap1GAP2 (total RG2), Slp1 (total Slp1), and Rab27 (total Rab27), each as 2% input. Vertical lines have been inserted to indicate a repositioned gel lane in the first and second panels from top as well as in the bottom panel. (D) Colocalization of transfected Rap1GAP2, Slp1, and Rab27a. Colocalization of EGFP-tagged Rab27a, VSV-tagged Rap1GAP2, and myc-tagged Slp1 overexpressed in HeLa cells was analyzed by immunofluorescence as described in “Confocal microscopy.” Arrows represent colocalization of all 3 proteins.
Involvement of Slp1 and Rap1GAP2 in platelet dense granule secretion
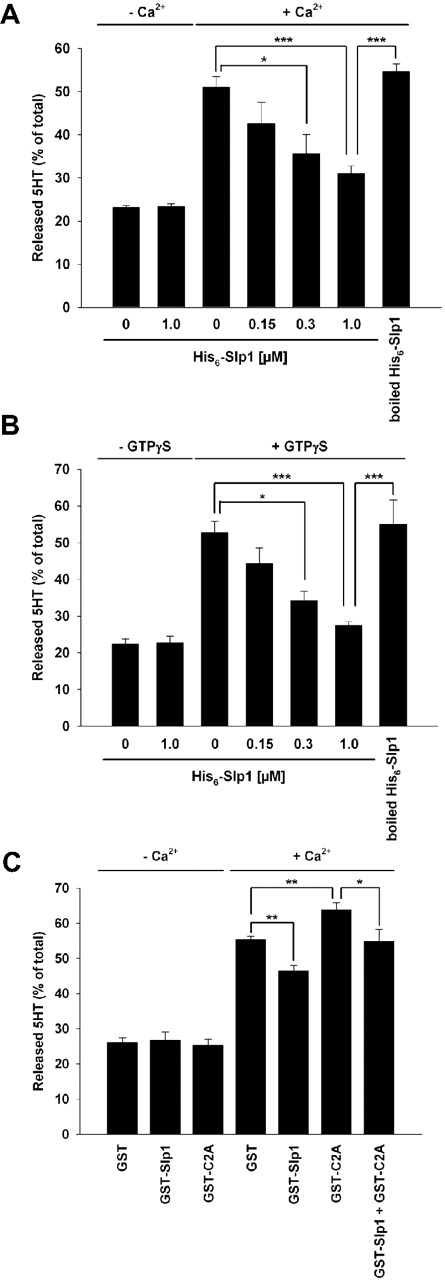
In platelets, Rab27 has been shown to regulate dense granule secretion.8 Slp1 is a Rab27-binding protein and thus might be a Rab27 effector. In addition, here we show that Slp1 also binds to Rap1GAP2. To elucidate the roles of Slp1 and Rap1GAP2 in granule secretion, we adopted and modified a previously described method for measuring serotonin release using streptolysin-O-permeabilized platelets.17,23,24 To induce granule release, we used calcium ions or the nonhydrolysable GTP-analog guanosine 5′-O-[gamma-thio]triphosphate (GTP-γS). On incubation of permeabilized platelets with purified recombinant Slp1, Ca2+-induced dense granule secretion of serotonin was significantly inhibited (Figure 5A). The inhibitory effect of Slp1 was dose-dependent, whereas baseline serotonin release was not affected by Slp1 (Figure 5A). Similar results were obtained in case of stimulation of permeabilized platelets with GTP-γS (Figure 5B). Boiling of the protein abolished the inhibitory effect of Slp1. To analyze the inhibitory function of Slp1 in greater detail, we studied the effects of the isolated C2A domain of Slp1 on dense granule release. Previous investigators have shown that the C2A domain competes with endogenous Slp1 and thus can be used as a dominant negative control.25,26 As shown in Figure 5C, the isolated C2A domain of Slp1 fused to GST augmented serotonin secretion. Furthermore, a molar excess of C2A reversed the inhibitory function of GST-Slp1. We used GST-fused proteins in these experiments because hexahistidine-tagged and untagged C2A proteins were not stable. Taken together, our data strongly support the concept that Slp1 inhibits dense granule secretion in platelets.
Slp1 inhibits platelet dense granule secretion in a dose-dependent manner. (A) Ca2+-induced dense granule secretion after incubation of permeabilized platelets with Slp1. Permeabilized platelets were incubated with the indicated concentrations of purified recombinant His6-tagged Slp1 or 1 μM boiled Slp1 and then stimulated with Ca2+ for 1 minute. For baseline serotonin secretion, platelets were left unstimulated in the absence or presence of Slp1. Baseline and Ca2+-induced secretion of dense granules was analyzed by measuring released serotonin (5-HT) as described in “Assay for secretion of platelet dense granules.” The results shown are expressed as mean ± SEM of 3 independent experiments performed in triplicate. *P < .05 (statistically significant); ***P < .001 (statistically significant). (B) GTP-γS–induced dense granule secretion after incubation of permeabilized platelets with Slp1. Permeabilized platelets were incubated with the indicated concentrations of purified recombinant His6-tagged Slp1 or 1 μM boiled Slp1 and then stimulated with GTP-γS for 5 minutes. Baseline and GTP-γS–induced secretion of dense granules was analyzed by measuring released serotonin (5-HT). The results shown are mean ± SEM of 3 independent experiments performed in triplicate. *P < .05 (statistically significant); ***P < .001 (statistically significant). (C) Ca2+-induced dense granule secretion after incubation of permeabilized platelets with the C2A domain of Slp1. Permeabilized platelets were incubated with 1 μM GST as control, GST-Slp1, GST-C2A, or with a combination of 1 μM GST-Slp1 and 5 μM GST-C2A and then incubated without or with Ca2+ for 1 minute. Baseline and Ca2+-induced secretion of dense granules was analyzed by measuring released serotonin (5-HT). The results shown are expressed as mean ± SEM of 6 independent experiments performed in triplicate. *P < .05 (statistically significant); **P < .01 (statistically significant).
Slp1 inhibits platelet dense granule secretion in a dose-dependent manner. (A) Ca2+-induced dense granule secretion after incubation of permeabilized platelets with Slp1. Permeabilized platelets were incubated with the indicated concentrations of purified recombinant His6-tagged Slp1 or 1 μM boiled Slp1 and then stimulated with Ca2+ for 1 minute. For baseline serotonin secretion, platelets were left unstimulated in the absence or presence of Slp1. Baseline and Ca2+-induced secretion of dense granules was analyzed by measuring released serotonin (5-HT) as described in “Assay for secretion of platelet dense granules.” The results shown are expressed as mean ± SEM of 3 independent experiments performed in triplicate. *P < .05 (statistically significant); ***P < .001 (statistically significant). (B) GTP-γS–induced dense granule secretion after incubation of permeabilized platelets with Slp1. Permeabilized platelets were incubated with the indicated concentrations of purified recombinant His6-tagged Slp1 or 1 μM boiled Slp1 and then stimulated with GTP-γS for 5 minutes. Baseline and GTP-γS–induced secretion of dense granules was analyzed by measuring released serotonin (5-HT). The results shown are mean ± SEM of 3 independent experiments performed in triplicate. *P < .05 (statistically significant); ***P < .001 (statistically significant). (C) Ca2+-induced dense granule secretion after incubation of permeabilized platelets with the C2A domain of Slp1. Permeabilized platelets were incubated with 1 μM GST as control, GST-Slp1, GST-C2A, or with a combination of 1 μM GST-Slp1 and 5 μM GST-C2A and then incubated without or with Ca2+ for 1 minute. Baseline and Ca2+-induced secretion of dense granules was analyzed by measuring released serotonin (5-HT). The results shown are expressed as mean ± SEM of 6 independent experiments performed in triplicate. *P < .05 (statistically significant); **P < .01 (statistically significant).
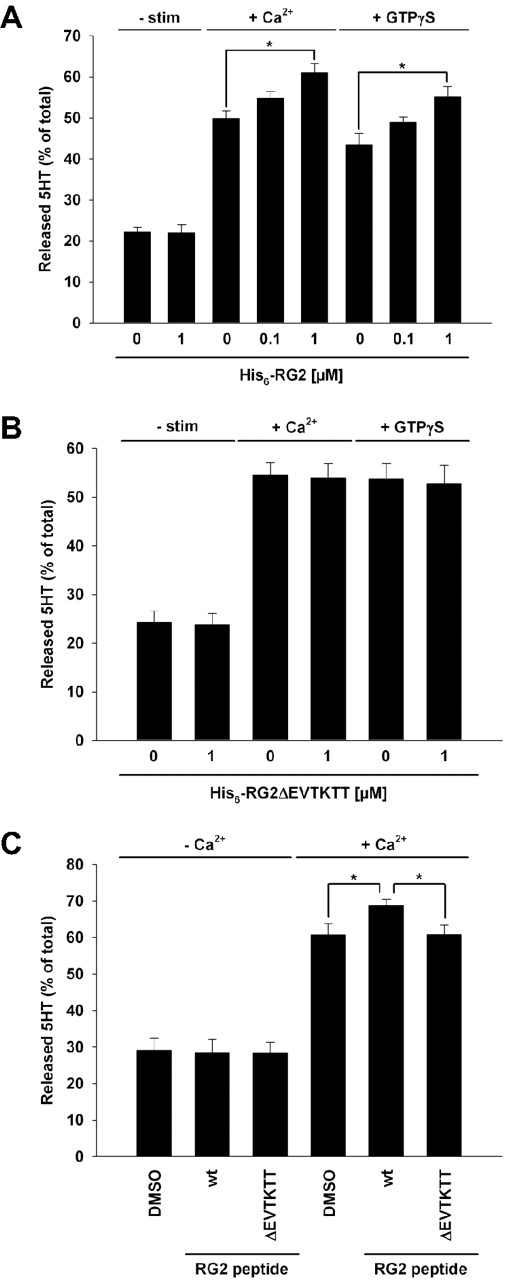
Next, we investigated a potential influence of Rap1GAP2 on secretion. Addition of purified recombinant wild-type Rap1GAP2 to permeabilized platelets significantly augmented Ca2+- and GTP-γS–induced dense granule secretion (Figure 6A). This effect was dose-dependent, and baseline levels of serotonin release were not changed by Rap1GAP2 (Figure 6A). Of note, addition of Rap1GAP2 together with Slp1 did not affect the inhibitory effect of Slp1 on dense granule secretion in the permeabilized cell system (data not shown). To investigate the relevance of the Rap1GAP2/Slp1 interaction, we prepared and purified a Rap1GAP2ΔEVTKTT mutant, which lacks the Slp1-binding site and therefore is not able to bind to Slp1 (Δ522-527 in Figure 3B). Incubation of permeabilized platelets with this mutant had no effect on Ca2+- and GTP-γS–induced dense granule secretion (Figure 6B). To corroborate the Rap1GAP2 effect, we also tested Rap1GAP2 peptides described in Figure 3E in the secretion assay system. The wild-type peptide containing the Slp1-binding motif, but not the peptide lacking this motif, augmented serotonin secretion from platelet-dense granules (Figure 6C). To assure Rap1GAP2 function in platelet secretion to be independent of its GAP activity, we incubated permeabilized platelets with purified recombinant Rap1 either native or GTP-loaded. As expected, no effect on serotonin secretion of platelet dense granules could be observed (Figure 7A). In addition, Slp1 binding to Rap1GAP2 had no effect on the catalytic GTPase-activating function of Rap1GAP2 in in vitro GAP assays using purified proteins (Figure 7B). From these data, we conclude that Slp1 inhibits platelet dense granule secretion, whereas Rap1GAP2 has a modulatory function in secretion that requires binding to Slp1 but is independent of the GAP activity of Rap1GAP2.
Rap1GAP2 enhances platelet dense granule secretion by binding to Slp1. (A) Ca2+- and GTP-γS–induced dense granule secretion after incubation of permeabilized platelets with Rap1GAP2. Permeabilized platelets were incubated without or with the indicated concentrations of purified recombinant His6-tagged Rap1GAP2 and then stimulated with Ca2+ for 1 minute or with GTP-γS for 5 minutes. For baseline serotonin secretion, platelets were left unstimulated (−stim) in the absence or presence of Rap1GAP2. Baseline and induced secretion of dense granules was analyzed by measuring released serotonin (5-HT) as described in “Assay for secretion of platelet dense granules.” The results shown are expressed as mean ± SEM of 3 independent experiments performed in triplicate. *P < .05 (statistically significant). (B) Ca2+- and GTP-γS–induced dense granule secretion after incubation of permeabilized platelets with mutant Rap1GAP2 that is deficient in Slp1 binding. Permeabilized platelets were incubated without or with 1 μM purified recombinant His6-tagged Rap1GAP2ΔEVTKTT mutant, which does not bind to Slp1. Then, platelets were stimulated with Ca2+ for 1 minute or with GTP-γS for 5 minutes. For baseline serotonin secretion, platelets were left unstimulated (-stim) in the absence or presence of Rap1GAP2ΔEVTKTT. Baseline and induced secretion of dense granules was analyzed by measuring released serotonin (5-HT). The results shown are mean ± SEM of 7 independent experiments performed in triplicate. (C) Ca2+-induced dense granule secretion after incubation of permeabilized platelets with Rap1GAP2 peptides. Permeabilized platelets were incubated with Rap1GAP2 wild-type peptide (RG2wt peptide) or Rap1GAP2 peptide lacking the Slp1-binding TKXT motif (RG2ΔEVTKTT) at 100 μM each. The solvent dimethyl sulfoxide was used as control. Baseline and Ca2+-induced secretion of dense granules was analyzed by measuring released serotonin (5-HT). The results shown are mean ± SEM of 5 independent experiments performed in triplicate. *P < .05 (statistically significant).
Rap1GAP2 enhances platelet dense granule secretion by binding to Slp1. (A) Ca2+- and GTP-γS–induced dense granule secretion after incubation of permeabilized platelets with Rap1GAP2. Permeabilized platelets were incubated without or with the indicated concentrations of purified recombinant His6-tagged Rap1GAP2 and then stimulated with Ca2+ for 1 minute or with GTP-γS for 5 minutes. For baseline serotonin secretion, platelets were left unstimulated (−stim) in the absence or presence of Rap1GAP2. Baseline and induced secretion of dense granules was analyzed by measuring released serotonin (5-HT) as described in “Assay for secretion of platelet dense granules.” The results shown are expressed as mean ± SEM of 3 independent experiments performed in triplicate. *P < .05 (statistically significant). (B) Ca2+- and GTP-γS–induced dense granule secretion after incubation of permeabilized platelets with mutant Rap1GAP2 that is deficient in Slp1 binding. Permeabilized platelets were incubated without or with 1 μM purified recombinant His6-tagged Rap1GAP2ΔEVTKTT mutant, which does not bind to Slp1. Then, platelets were stimulated with Ca2+ for 1 minute or with GTP-γS for 5 minutes. For baseline serotonin secretion, platelets were left unstimulated (-stim) in the absence or presence of Rap1GAP2ΔEVTKTT. Baseline and induced secretion of dense granules was analyzed by measuring released serotonin (5-HT). The results shown are mean ± SEM of 7 independent experiments performed in triplicate. (C) Ca2+-induced dense granule secretion after incubation of permeabilized platelets with Rap1GAP2 peptides. Permeabilized platelets were incubated with Rap1GAP2 wild-type peptide (RG2wt peptide) or Rap1GAP2 peptide lacking the Slp1-binding TKXT motif (RG2ΔEVTKTT) at 100 μM each. The solvent dimethyl sulfoxide was used as control. Baseline and Ca2+-induced secretion of dense granules was analyzed by measuring released serotonin (5-HT). The results shown are mean ± SEM of 5 independent experiments performed in triplicate. *P < .05 (statistically significant).
Slp1 and Rap1GAP2 effects on platelet secretion are not mediated by Rap1. (A) Ca2+-induced dense granule secretion after incubation of permeabilized platelets with Rap1. Permeabilized platelets were incubated with 1 μM of either BSA as control or purified recombinant native Rap1b or Rap1b loaded with GTP. Then, platelets were stimulated with Ca2+ for 1 minute. Baseline and Ca2+-induced secretion of dense granules was analyzed by measuring released serotonin (5-HT). The results shown are mean ± SEM of 5 independent experiments performed in triplicate. (B) In vitro GAP assay. Epitope-tagged Slp1, Rap1GAP2, and Rap1GAP2 in complex with Slp1 were expressed in HeLa cells and purified using tag-specific affinity agarose. In parallel, His6-tagged Rap1b was purified from E coli and loaded with [32P]-GTP. Precipitated Slp1 and Rap1GAP2 proteins were added to the GTP-loaded Rap1b, and reactions were incubated at 25°C. Aliquots were removed at the indicated time points, and amounts of released [32P] were determined by liquid scintillation counting and plotted as a percentage of input Rap1b-bound [32P]-GTP counts. Data are the mean ± SE of 3 independent experiments performed in triplicate.
Slp1 and Rap1GAP2 effects on platelet secretion are not mediated by Rap1. (A) Ca2+-induced dense granule secretion after incubation of permeabilized platelets with Rap1. Permeabilized platelets were incubated with 1 μM of either BSA as control or purified recombinant native Rap1b or Rap1b loaded with GTP. Then, platelets were stimulated with Ca2+ for 1 minute. Baseline and Ca2+-induced secretion of dense granules was analyzed by measuring released serotonin (5-HT). The results shown are mean ± SEM of 5 independent experiments performed in triplicate. (B) In vitro GAP assay. Epitope-tagged Slp1, Rap1GAP2, and Rap1GAP2 in complex with Slp1 were expressed in HeLa cells and purified using tag-specific affinity agarose. In parallel, His6-tagged Rap1b was purified from E coli and loaded with [32P]-GTP. Precipitated Slp1 and Rap1GAP2 proteins were added to the GTP-loaded Rap1b, and reactions were incubated at 25°C. Aliquots were removed at the indicated time points, and amounts of released [32P] were determined by liquid scintillation counting and plotted as a percentage of input Rap1b-bound [32P]-GTP counts. Data are the mean ± SE of 3 independent experiments performed in triplicate.
Discussion
We have identified Slp1 as new direct binding partner of Rap1GAP2 in platelets, and we have shown that Slp1 is involved in the regulation of platelet dense granule secretion.
Rap1GAP2 is a modular protein containing a central GAP domain that is required to confer GTPase activity toward Rap1, a protein in control of platelet aggregation. However, the large C-terminal region of Rap1GAP2 has so far been of unknown function. In our present study, we demonstrated that at least part of this C-terminal region of Rap1GAP2 is involved in protein-protein interactions. We mapped the Slp1-binding site to a very small motif composed of 3 essential residues in the C-terminus of Rap1GAP2. This TKXT motif interacts with the C2A domain of Slp1 that has been shown to mediate binding of Slp1 to the plasma membrane.27 C2 domains are generally considered to mediate phospholipid binding20 ; however, certain C2 domains have also been observed to be involved in protein-protein interactions. These interactions usually occur intramolecularly, such as the interaction of the C1 and C2 domains of PKC28 or the binding of the C2 domain to the catalytic domain in SynGAP.29 The C2 domain of PKCδ was recently shown to bind to phosphotyrosine residues.30 Although the TKXT motif of Rap1GAP2 contains 2 threonines that could be subjected to phosphorylation, our data suggest that phosphorylation is not required for binding of the C2A domain to the TKXT motif in Rap1GAP2 (Figure 3D). The TKXT motif could be involved in subcellular targeting of Rap1GAP2 to the plasma membrane via Slp1, and we indeed observed a colocalization of Rap1GAP2 and Slp1 at the plasma membrane (Figure 4D).
Slp1 was previously shown to stimulate secretion of prostate-specific antigen by prostate cells25 and secretion of azurophilic granules by granulocytes.26 Furthermore, Slp1 regulates exocytosis of secretory lysosomes by cytotoxic T lymphocytes31 and blocks amylase secretion by pancreatic acinar cells.32 For the first time, we show that Slp1 is also expressed in human platelets. Slp1 is a member of a family of Rab27-binding proteins and is known to interact with Rab27 via an N-terminal Rab27-binding domain.33,34 We could verify binding of Slp1 to Rab27 in human platelets and showed that Rap1GAP2 can join this complex (Figure 4C). However, possible regulatory mechanisms involved in the formation of the Slp1/Rap1GAP2/Rab27 protein complex in platelets will be the subject of future studies.
To elucidate the functional role of the Slp1/Rap1GAP2 interaction in platelets, we performed secretion assays using streptolysin-O–permeabilized platelets. Addition of recombinant Slp1 strongly reduced serotonin secretion. Experiments using the isolated membrane-binding C2A domain of Slp1 as dominant-negative control25,26 showed the opposite effect, thus confirming the inhibitory role of full-length Slp1. Interestingly, the Slp1 effect was independent of the stimulus; both Ca2+- and GTP-γS–induced secretion was inhibited. Ca2+ probably triggers the final fusion event of granules with the plasma membrane, whereas the GTP-γS effect might be mediated by small GTPases, such as Rab27 or Ral.9,35 Thus, Slp1 appears to be involved in a phase of the secretion process common to both stimuli. Studies of other Slp family members suggest that Slps can have dual, stimulatory and inhibitory, roles in granule secretion through diverse interactions with other proteins or the membrane.36-40 Testing of the Slp1-interacting protein Rap1GAP2 in secretion assays with permeabilized platelets revealed that Rap1GAP2 augments dense granule release. This effect was dependent on the binding of Rap1GAP2 to Slp1 and could be mimicked by a small peptide containing the Slp1-binding motif. Although Rap1GAP2 is required for GTPase activity of Rap1, Rap1 itself does not have any direct effect on platelet dense granule secretion.
In conclusion, Slp1 inhibits dense granule secretion in platelets. Furthermore, Slp1 interacts with Rap1GAP2, and binding of Rap1GAP2 to Slp1 augments dense granule release. The effect of Rap1GAP2 on secretion requires the new Slp1-binding TKXT protein motif in the C-terminal part of Rap1GAP2. Our data provide new insights into the control of granule secretion and suggest possible connections between the regulation of granule secretion and aggregation in platelets.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank S. Bhakdi for the generous gift of streptolysin-O, M. C. Seabra for providing Rab27 constructs, O. Danielewski for help in yeast-2-hybrid screening, W. Müller-Esterl for support throughout the course of this work, and all our colleagues from the Institute of Biochemistry II for help and discussions.
This work was supported by the Deutsche Forschungsgemeinschaft (grant SFB 553), the Excellence Cluster Cardio-Pulmonary System, and the Science Foundation Ireland (Principal Investigator Program grant).
Authorship
Contribution: O.N., M.H., J.B., C.P., K.G., and A.P.S. performed experiments and analyzed data; O.N., M.H., and A.P.S. designed the research; and O.N. and A.P.S. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Albert P. Smolenski, School of Medicine and Medical Science, UCD Conway Institute of Biomolecular and Biomedical Research, University College Dublin, Belfield, Dublin 4, Ireland; email: albert.smolenski@ucd.ie.



![Figure 7. Slp1 and Rap1GAP2 effects on platelet secretion are not mediated by Rap1. (A) Ca2+-induced dense granule secretion after incubation of permeabilized platelets with Rap1. Permeabilized platelets were incubated with 1 μM of either BSA as control or purified recombinant native Rap1b or Rap1b loaded with GTP. Then, platelets were stimulated with Ca2+ for 1 minute. Baseline and Ca2+-induced secretion of dense granules was analyzed by measuring released serotonin (5-HT). The results shown are mean ± SEM of 5 independent experiments performed in triplicate. (B) In vitro GAP assay. Epitope-tagged Slp1, Rap1GAP2, and Rap1GAP2 in complex with Slp1 were expressed in HeLa cells and purified using tag-specific affinity agarose. In parallel, His6-tagged Rap1b was purified from E coli and loaded with [32P]-GTP. Precipitated Slp1 and Rap1GAP2 proteins were added to the GTP-loaded Rap1b, and reactions were incubated at 25°C. Aliquots were removed at the indicated time points, and amounts of released [32P] were determined by liquid scintillation counting and plotted as a percentage of input Rap1b-bound [32P]-GTP counts. Data are the mean ± SE of 3 independent experiments performed in triplicate.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/114/7/10.1182_blood-2008-05-155234/4/m_zh89990940260007.jpeg?Expires=1765903988&Signature=4C~5FhyrEaMHx2z16JCp9UT2uA0IzykSJasi2DxLczDcOyF-IL4Fjo~kIf1sF1Epg-V4EeS~r11wraqiKGxku~IJM1FpWBzSlUsoPu4gQZDJ2Uom9z0Arl-NE8yjz3JMQjz5SsrjLP~tHUGWeS2mrexIXcLomF3qWXBz3DHzwLTdk0z7NJVmUE7XCUjr42MeZ4JmkQydJTYrQuzdGbVo5aiN1m3~qBZQ-5j3WykqRZLqQuJiiHeKHLBMJ~yyz-gsoIbFVZQyPx1EuLLbHN1GOZZ7eUqS71empw-S7v0gSwmzBPv38tB7xOKfdDDy3Q2ClFADsIGwasbYk~rtVT3fqA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal