Abstract
Chemokines mediate the signaling and migration of T cells, but little is known about the transcriptional events involved therein. Microarray analysis of CXC chemokine ligand (CXCL) 12−treated T cells revealed that Wnt ligands are significantly up-regulated during CXCL12 treatment. Real-time polymerase chain reaction and Western blot analysis confirmed that the expression of noncanonical Wnt pathway members (eg, Wnt5A) was specifically up-regulated during CXCL12 stimulation, whereas β-catenin and canonical Wnt family members were selectively down-regulated. Wnt5A augmented signaling through the CXCL12-CXCR4 axis via the activation of protein kinase C. Moreover, Wnt5A expression was required for CXCL12–mediated T-cell migration, and rWnt5A sensitized human T cells to CXCL12-induced migration. Furthermore, Wnt5A expression was also required for the sustained expression of CXCR4. These results were further supported in vivo using EL4 thymoma metastasis as a model of T-cell migration. Together, these data demonstrate that Wnt5A is a critical mediator of CXCL12-CXCR4 signaling and migration in human and murine T cells.
Introduction
Chemokines are homologous chemotactic proteins that interact with G protein–coupled, 7-transmembrane receptors.1 Chemokines may be inflammatory or homeostatic, and facilitate lymphocyte migration during inflammation and immune surveillance.2-4 Receptor activation leads to a cascade of cellular activation, including a variety of intracellular signaling pathways that regulate the trafficking of cells. CXC chemokine receptor (CXCR) 4, a chemokine receptor specific for the CXC chemokine ligand (CXCL) 12, plays a key role in the retention of stem cells, differentiating B cells and neutrophils within bone marrow, and B-cell positioning within lymph nodes.5,6 CXCL12 acts as a strong chemoattractant during inflammation and is expressed in a variety of tissues.7 In 2001, Suzuki et al8 revealed that genes associated with DNA repair, detoxification, apoptosis, cell morphology, cell adhesion, and signal transduction were up-regulated in CD4+ T cells upon CXCL12 stimulation. This up-regulation correlated with the ability of CXCL12 to promote CD4+ T-cell survival and prime T cells for cellular activation, increasing their ability to respond to and survive immunologic challenges. Despite this report, the molecular changes induced by CXCL12-CXCR4 interactions and their effects on receptor-mediated signaling, migration, and function in T cells are largely unknown.
To better understand the pathways by which CXCL12 mediates the activation and migration of T cells, we performed microarray analysis on CXCL12-treated human T cells. These analyses have revealed a critical role for the noncanonical Wnt molecule, Wnt5A, in CXCR4-mediated signaling and function in human and murine T cells. The Wnt family of proteins are secreted glycoproteins,9 which signal via their cognate receptors, the Frizzled (Fzd) family of receptors. The interactions between specific Wnts and Fzds dictate which G proteins are activated and which signals are transduced downstream.10 Recent studies by Wu et al11 have demonstrated that endothelial cell–derived Wnts, primarily Wnt3A, induce matrix metalloproteinase 2 and 9 expression in effector T cells. Wnt-Fzd and chemokine signaling pathways appear to cooperate and control cell polarity and directional movement of melanoma cells.12 In this work, we study the relevance of the noncanonical Wnt signaling pathway in T-cell polarity, cellular trafficking, and CXCR4 function.
Methods
Reagents and supplies
Recombinant CXCL12 (stromal cell–derived factor-1α) was purchased from PeproTech. Polyclonal antibodies against Fzd2 and Wnt5A, as well as rWnt5A, were obtained from R&D Systems. Antibodies for β-catenin and CXCR4 were obtained from Cell Signaling Technology. The protein kinase C (PKC)–specific blocker GO6983 (used at 1 μM) and the pharmacologic blocker of CXCR4, AMD3100 (used at 1 μM), were purchased from Sigma-Aldrich.
Rac activation assay
Activated Rac1 was assayed using the Rac activation assay kit obtained from Cytoskeleton. Briefly, cells were treated with 100 ng/mL CXCL12 for 15 minutes, then washed twice with ice-cold phosphate-buffered saline (PBS), and lysed in lysis buffer containing protease inhibitors. Total protein (500 μg) was used to immunoprecipitate activated Rac1 using PAK1-PBD (p21-activating kinase 1–p21 binding domain) beads by incubation for 16 hours at 4°C. The beads were collected from mixture by centrifugation at 500g and washed 4 times. A total of 30 μL sodium dodecyl sulfate sample buffer was added to the beads, and samples were subjected to Western blot analysis using anti-Rac1 antibody (1:500; Cytoskeleton). Whole-cell lysate (25 μg) was used for Western blot analysis of total Rac1.
Cell cultures and cell lines
CEM, a human lymphoblastoma cell line, was purchased from the ATCC. EL4 lymphoma cells were chosen due to their ability to form liver metastases in vivo. Cell lines were maintained and propagated in RPMI 1640 medium with 2 mM l-glutamine, 4.5 g/L glucose, 10 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), 1.0 mM sodium pyruvate, 10% heat-inactivated fetal calf serum, and 100 U/mL penicillin-streptomycin.
Human T-cell isolation and culture
Peripheral blood mononuclear cells were isolated from healthy patient volunteers (under Institutional Review Board protocol NIA2003-054, with informed consent obtained in accordance with the Declaration of Helsinki), using a Ficoll-Paque (Amersham Biosciences) density gradient centrifugation, followed by treatment with ammonium chloride lysis solution (Biofluids) to eliminate the remaining erythrocytes. The isolated cells were washed twice in PBS and resuspended in column buffer (R&D Systems). T cells were isolated by negative selection using enrichment columns, according to the manufacturer's instructions (R&D Systems). Cells were typically more than 97% pure, as assessed by fluorescence-activated cell sorting (FACS). Purified primary T cells were suspended in RPMI 1640 medium with 2 mM l-glutamine, 10 mM HEPES, 1.0 mM sodium pyruvate, 10% heat-inactivated fetal calf serum, and 100 U/mL penicillin-streptomycin; stored overnight at 4°C; then incubated at 37°C for 1 hour, followed by treatment with chemokines or rWnt5A, as described.
Microarray analysis
Primary T cells from 3 different donors were treated with human CXCL12 (PeproTech) at 100 ng/mL for 12 hours in a humidified incubator at 37°C with 5% CO2. Control cells from the same 3 donors were incubated in media only. Cells were harvested and washed twice with ice-cold PBS, followed by isolation of RNA and preparation of cDNA and cRNA. The cRNA was amplified and labeled with either Cy-3 or Cy-5, using the Agilent low-input linear amplification kit, and hybridized to human 44K whole genome oligo array slides (Agilent). Each donor sample was applied to a single array (total of 6 arrays: 3 donor samples treated, 3 untreated). Images were analyzed using the Agilent Feature Extractor Software, version A.7.5.1, and ratios for each spot were calculated.
Data mining and analysis
Final analysis was performed using the Biometric Research Branch–Array Tool developed by National Cancer Institute, a National Human Genome Research Institute in-house suite of software and software developed by National Cancer Institute and Stanford University. Raw data were z-transformed, normalized (median more than entire array), and filtered. Data have been deposited to the GEO database under accession number GSE12449 (http://www.ncbi.nlm.nih.gov/projects/geo/query/acc.cgi?acc=GSE12449). Web-based functional clustering on genes with a fold change of 2.5 or greater was performed using SOURCE DATABASE developed by Stanford University (www.ingenuity.com) and web-based software developed by the National Cancer Institute (Biometric Research Branch Array Tool).
Chemotaxis assay
T cells were labeled with 5 μM calcein (Molecular Probes), followed by treatments, as described. A total of 100 000 to 200 000 cells was placed into the upper chamber of a Transwell chemotaxis chamber (Corning Life Sciences), and CXCL12 was placed into the lower chamber at 100 ng/mL. For thymus and activation regulated chemokine (TARC) and C-C chemokine ligand (CCL) 19 assays, chemokines were placed in the lower chamber at 250 ng/mL. Chambers were incubated for 2 hours in a CO2 incubator at 37°C with 5% humidity. The amount of migrated cells in the lower chamber was assayed by reading the calcein fluorescence using a fluorometer (Fluoroskan Ascent FL; Labsystems). Cell migration was expressed in terms of percentage of migration index, as follows: percent migration index = (fluorescence of migrated cells/fluorescence of total input cells) × 100.
Small interfering RNA transfections
Primary human T cells and CEM cells were transfected with Wnt5A small interfering RNA (siRNA; Dharmacon) or a negative control siRNA. The siRNAs used consist of a mixture of several antisense oligonucleotides sequence known as On-Target Plus-Smartpool. The siRNA sequences of Wnt5A are as follows: first duplex, sense strand, GUUCAGAUGUCAGAAGUAUUU, and antisense strand, 5′-P-AUACUUCUGACAUCUGAACUU; second duplex, sense strand, UCAGAUGUCAGAAGU-AUAUUU, and antisense strand, 5′-P-AUAUACUUCUGACAUUCUGAUU; third duplex, sense strand, GCGACAACAUCGACUAUGGUU, and antisense strand, 5′-P-CCAUAGUCGAUGUUGUCGCUU; and fourth duplex, sense strand, GGUCGCUAGGUAUGAAUAAUU, and antisense strand, 5′-P-UUAUUCAUACCUAGCGACCUU. Fzd2 sequences are listed under catalog M-005501-01 (Dharmacon). The siRNA was electroporated into primary and established cells using the human T-cell nucleofector transfection kit (VPA-1002 and VPA-1003; Amaxa Biosystem), according to the manufacturer's protocol. In brief, control and gene-specific siRNA were dissolved in siRNA solution buffer at 1 mg/mL. Two million cells were electroporated in 100 μL Amaxa transfection buffer (human T-cell nucleofector buffer; Amaxa), with 2 μg siRNA, on the U-014 setting for primary T cells and the X-001 setting for CEM cells. The cells were immediately suspended into prewarmed culture media after transfection. Inhibition of gene expression was tested by both real-time polymerase chain reaction (PCR) and immunoblot analysis.
Development of stably transfected cell lines
The plasmid-containing vectors (PLKO.1 backbone) expressing short hairpin RNA (shRNA) for Wnt5A and its details can be obtained from the company website (Origene). For Wnt5A overexpression, Wnt5A cDNA was cloned into a pDEST12.2 vector (Invitrogen), and sequenced to confirm accurate cloning. All plasmids were propagated in Escherichia coli, and purified plasmid was transfected into CEM cell lines, as described above. Transfected cells were cultured in RPMI 1640 with 10% fetal calf serum, 1 mM HEPES, and the recommended dose of penicillin-streptomycin. Stable transfectants were selected by the addition of 2 μg/mL puromycin (PLKO.1) or 1 mg/mL G418 (pDEST12.2), and then tested for Wnt5A status by real-time PCR and immunoblot analysis.
Immunoblotting
Cells were lysed in radioimmunoprecipitation assay buffer obtained from Santa Cruz Biotechnology. A total of 50 μg protein was run on a 4% to 12% gradient or 10% fixed readymade gel (NuPage), and then transferred onto 2-μm polyvinylidene difluoride membrane. Membranes were blocked with 5% milk for 1 hour and incubated in primary antibody overnight at 4°C. The next day, the membrane was washed 3 times with 1× Tris-buffered saline with Tween 20 buffer (Invitrogen) for 5 minutes each, followed by incubation in a secondary antibody for 1 hour at room temperature. Bands were visualized using enhanced chemiluminescence solution (Amersham).
FACS
FACS analysis was used to analyze the status of CXCR4, C-C chemokine receptor (CCR)4, or CCR7 in different cell lines and primary T cells. Cells (5 × 105 cells per sample) were stained with 1 μg anti-CXCR4, anti-CCR4, or anti-CCR7 monoclonal antibody for 45 minutes at 4°C. In parallel, the cells were incubated with mouse immunoglobulin G as a control. After incubation, the cells were washed 3 times and then incubated with anti−mouse phycoerythrin-conjugated secondary antibody (BD Pharmingen) for 30 minutes at 4°C, after which the samples were washed and examined using a flow cytometer (FACSCan; BD Biosciences).
Real-time PCR
A 96-well plate–based assay containing primers for genes involved in the Wnt-Fzd pathway was purchased from SuperArray Biosciences. The detailed procedure can be obtained from www.superarray.com. T cells from 4 donors were treated with 100 ng/mL CXCL12 for 12 hours, and total RNA was isolated. The cDNA was prepared from 500 ng RNA using a cDNA preparation kit (Bio-Rad). All reagents for the real-time PCR, including the Sybrgreen, were purchased from Bio-Rad. The plates were run on an ABI 7500 real-time PCR machine (Applied Biosystems). Data were analyzed using software supplied by SuperArray Biosciences. Statistical analysis was performed using the Student t test, and the data were represented as the mean value of 4 donors plus or minus standard error.
Preparation of primary T cells from mice
Null mutant mice for CXCR4 with C57BL/6 background were purchased from The Jackson Laboratory. The genetic status of CXCR4 was confirmed by genotyping. Sacrifice of mice and isolation of lymph nodes from CXCR4-null mutant mice were performed, according to National Institutes of Health protocols 272-LI-2007 and 343-LI-2007, respectively, and were approved by the National Institutes of Health institutional review board. Mice were euthanized using CO2 according to animal care and use committee guidelines governing CO2-induced rodent euthanasia. Mice were dissected, and spleens were isolated and stored in ice-cold RPMI 1640 with 10% fetal calf serum and 100 U/mL penicillin-streptomycin. Lymph nodes from axillaries and inguinal zone were also isolated in the same media. T cells were isolated using the mouse T-cell enrichment column (R&D Systems), kept overnight at 4°C, and then incubated at 37°C for 1 hour, followed by treatment with mouse 100 ng/mL CXCL12 (PeproTech) or rWnt5A at 400 ng/mL dose and at a concentration of 106/mL.
Statistical analysis
All the data were always repeated in triplicate at least. The Student t test was used for statistical analysis, and a P value less than .05 was considered as significant wherever applicable.
In vivo metastasis assays and immunohistochemistry
A total of 2 × 105 EL4 murine thymoma cells was treated with 0.05 μg/mL rWnt5A protein for 16 hours, 0.02 μg/mL CXCL12 (experimental groups), or untreated (control group), and then injected into the tail vein of 8-week-old C57BL/6 mice (The Jackson Laboratory). The experimental groups received biweekly injections of rWnt5A (75 ng/mouse) or CXCL12 (30 ng/mouse) starting the third day after primary tumor cell inoculation. Twenty-one days after the inoculation of cells, livers were removed, fixed in paraformaldehyde, and embedded in paraffin. Antigen retrieval was performed as previously described.13 For CD3 staining, we used an alkaline phosphatase substrate kit from Vector Laboratories, in which tissues are blocked in 1% bovine serum albumin for 15 minutes, probed with 1.35 μg/mL CD3 antibody (BD Pharmingen) at 4°C for overnight, then washed and probed with alkaline phosphatase-conjugated anti−hamster immunoglobulin G, followed by alkaline phosphatase development. For CD3 and Wnt5A costaining, tissues were blocked as described, followed by an overnight incubation at 4°C in 2.5 μg/mL biotinylated Wnt5A (R&D Systems) and 1.35 μg/mL CD3 antibody. The following day, the cells were washed and then probed with a streptavidin-horseradish peroxidase–conjugated secondary antibody, washed, and followed by diaminobenzidine chromagen, as well as alkaline phosphatase development of the CD3 antibody, as described above. Cells were dehydrated through an ethanol/xylene series, as previously described,13 and coverslipped and imaged. No counterstain was used so as not to mask the alkaline phosphatase staining.
Results
Microarray analysis of CXCL12-stimulated T cells reveals changes in noncanonical Wnt signaling
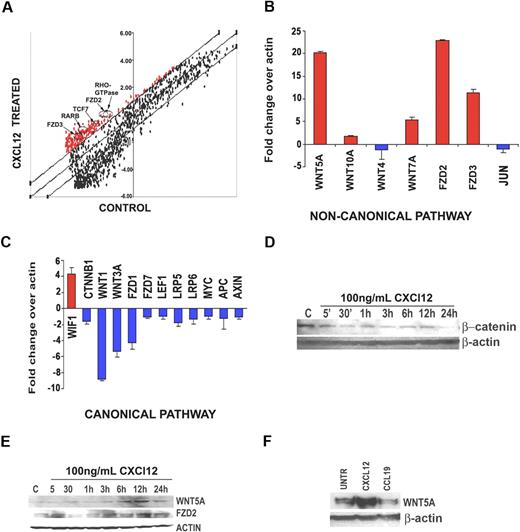
Resting human T cells from 3 separate donors were treated with either CXCL12 or media alone. Microarray analysis was conducted on each donor, and genes with greater than 2.5-fold change across all experimental compared with control conditions were considered significant. The largest increases in gene expression occurred in members of the Wnt pathway, including Fzd receptors 2 and 3, TCF7, and others (Figure 1A arrows). To confirm this observation, we used RT-2 profiler real-time PCR plates that contain up to 90 genes specific to a single pathway, in this case, the Wnt pathway. These data demonstrated a very striking pattern of Wnt expression in the CXCL12-treated T cells. Noncanonical Wnts such as WNT5A, WNT10A, and WNT7A were all significantly up-regulated (Figure 1B), and canonical Wnts such as WNT3A and the key mediator of the canonical pathway, β-catenin (CTNNB1), were down-regulated (Figure 1C) upon CXCL12 treatment. Fzd receptor expression was also influenced by chemokine stimulation. FZD2, involved in noncanonical Wnt signaling, was up-regulated, and the canonical Fzd receptors, including FZD1 and FZD7, were down-regulated. In addition, the Wnt inhibitory factor, WIF1, known to inhibit canonical Wnt signaling,14 was highly up-regulated, and other molecules in the canonical pathway, including APC, Axin, LEF, and MYC, were down-regulated (Figure 1C). Moreover, JUN, a mediator of the noncanonical Wnt planar cell polarity pathway,15 was also found to be down-regulated in our studies (Figure 1C). This suggested that the noncanonical Wnt pathway up-regulated was specifically the PKC/Ca2+ pathway. Given that this pathway is best characterized by WNT5A, we focused our further efforts on this member of the Wnt family. To determine whether the protein levels of Wnt ligands and their downstream targets changed, primary human T cells derived from 4 different donors were stimulated with CXCL12 at timepoints from 5 and 30 minutes to 1, 3, 6, 12, and 24 hours. β-Catenin protein expression did not increase upon CXCL12 treatment (Figure 1D), and in fact decreased. Manipulation of β-catenin levels did not appear to greatly affect T-cell migration (data not shown). Wnt5A expression, on the other hand, increased by 6 hours (Figure 1E), as did Fzd2 expression. Furthermore, this effect was specific to CXCL12, as CCL19 did not increase Wnt5A expression, perhaps even decreasing it slightly (Figure 1F).
Microarray analysis reveals up-regulation of noncanonical Wnt signaling in CXCL12-mediated T-cell migration. (A) Primary T cells from 3 different donors were treated with human CXCL12 at 100 ng/mL for 12 hours. Cells from the same 3 donors were incubated in media only as a control. RNA was extracted from each sample and subjected to microarray analysis (6 arrays total). Genes with a 2.5-fold cutoff (either up or down) were considered significantly changed. Genes involved in noncanonical Wnt signaling (arrows) were found to be significantly up-regulated (> 2.5-fold increase, P < .05) in the CXCL12-treated T-cell groups. (B) Primary T cells from 4 additional donors were treated with human CXCL12 at 100 ng/mL for 12 hours. Cells from these 4 donors were incubated in media only as a control. RNA was extracted and cDNA was used to perform real-time PCR analysis using the Open Biosystems Superarray pathway reverse transcription–PCR kits. Superarray reverse transcription–PCR analysis confirms that gene members of the noncanonical Wnt signaling pathway are up-regulated (B), whereas members of the canonical Wnt signaling pathway are down-regulated (C). All genes are normalized to actin. Interestingly, the only gene up-regulated in the canonical Wnt signaling pathway is the Wnt inhibitory factor-1 (WIF1), an inhibitor of canonical Wnt signaling. To validate the RNA results, Western blot analysis was performed. (D) Primary human T cells (pool of 3 donors) were treated with 100 ng/mL CXCL12 for the times indicated, then examined by Western blot analysis for β-catenin, Wnt5A, and Fzd2 expression. These data demonstrate that β-catenin is decreased by CXCL12 treatment, but Wnt5A and Fzd2 expression are increased (E). (F) Wnt5A up-regulation is specific to CXCL12, as Western blot analysis demonstrates that CCL19 (100 ng/mL, 12 hours) does not increase Wnt5A expression. Western blots were repeated 3 times, and a representative blot is shown.
Microarray analysis reveals up-regulation of noncanonical Wnt signaling in CXCL12-mediated T-cell migration. (A) Primary T cells from 3 different donors were treated with human CXCL12 at 100 ng/mL for 12 hours. Cells from the same 3 donors were incubated in media only as a control. RNA was extracted from each sample and subjected to microarray analysis (6 arrays total). Genes with a 2.5-fold cutoff (either up or down) were considered significantly changed. Genes involved in noncanonical Wnt signaling (arrows) were found to be significantly up-regulated (> 2.5-fold increase, P < .05) in the CXCL12-treated T-cell groups. (B) Primary T cells from 4 additional donors were treated with human CXCL12 at 100 ng/mL for 12 hours. Cells from these 4 donors were incubated in media only as a control. RNA was extracted and cDNA was used to perform real-time PCR analysis using the Open Biosystems Superarray pathway reverse transcription–PCR kits. Superarray reverse transcription–PCR analysis confirms that gene members of the noncanonical Wnt signaling pathway are up-regulated (B), whereas members of the canonical Wnt signaling pathway are down-regulated (C). All genes are normalized to actin. Interestingly, the only gene up-regulated in the canonical Wnt signaling pathway is the Wnt inhibitory factor-1 (WIF1), an inhibitor of canonical Wnt signaling. To validate the RNA results, Western blot analysis was performed. (D) Primary human T cells (pool of 3 donors) were treated with 100 ng/mL CXCL12 for the times indicated, then examined by Western blot analysis for β-catenin, Wnt5A, and Fzd2 expression. These data demonstrate that β-catenin is decreased by CXCL12 treatment, but Wnt5A and Fzd2 expression are increased (E). (F) Wnt5A up-regulation is specific to CXCL12, as Western blot analysis demonstrates that CCL19 (100 ng/mL, 12 hours) does not increase Wnt5A expression. Western blots were repeated 3 times, and a representative blot is shown.
Wnt5A directly affects the migration of T cells and is necessary for CXCL12-mediated T-cell migration
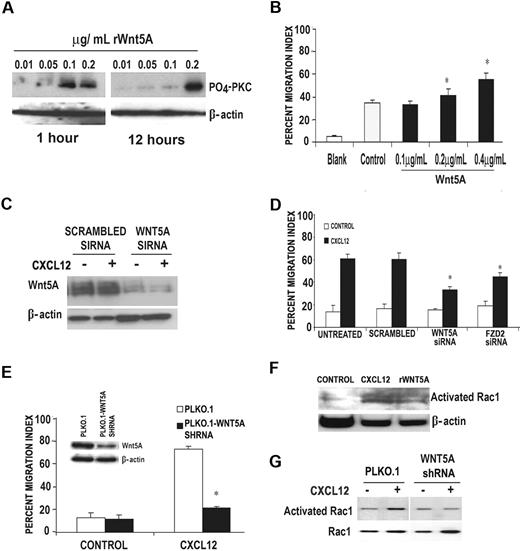
The above data beg the question whether Wnt5A is necessary for CXCL12-mediated T-cell migration, or whether its expression was merely an unrelated product of T-cell activation. T cells were examined for their ability to respond to rWnt5A at various time periods by measuring levels of phosphorylated PKC, an end point by which the efficacy of rWnt5A treatment can be measured.16 RWnt5A, at a concentration of 0.2 μg/mL, was able to activate PKC in human T cells in a biphasic manner at 1 hour and again at 12 hours (Figure 2A). Both rWnt5A and CXCL12 activated PKC in T cells, and PKC was required for CXCL activation of Wnt5A (supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Primary human T cells, pretreated with rWnt5A for 16 hours, were examined for their ability to migrate in response to CXCL12 using a Transwell migration system. Pretreatment of T cells with rWnt5A increased their ability to migrate in response to CXCL12, in a dose-dependent manner (Figure 2B). Knockdown of WNT5A using siRNA in primary human T cells could not be recovered by CXCL12 treatment (Figure 2C) and inhibited the ability of the T cells to migrate in response to CXCL12 by more than 50% (Figure 2D). Knockdown of the Wnt5A receptor FZD2 to approximately 30% of control (data not shown) also resulted in a significant inhibition of T-cell migration in response to CXCL12 (Figure 2D). This was also true when using the CEM T-cell line in which WNT5A is stably knocked down using a WNT5A shRNA (Figure 2E and inset). These data indicate that WNT5A expression is necessary for the migration of T cells in response to CXCL12.
RWnt5A can influence CXCL12-mediated signaling in primary T cells. (A) Western blot analysis demonstrates that phospho-PKC (α, β, and γ isoforms) is up-regulated in primary human T cells by treatment with 0.2 μg/mL rWnt5A for 16 hours. This activation occurs in a biphasic manner. (B) T cells pretreated with rWnt5A for 16 hours demonstrate greater migration in response to CXCL12 in a dose-dependent manner; n (donors) = 3, *P < .01. (C) Western blot analysis demonstrates that in the presence of WNT5A siRNA, 100 ng/mL CXCL12 (12 hours of treatment) failed to increase Wnt5A expression compared with control siRNA-transfected cells. Primary human T cells were then transfected with siRNA against either WNT5A or FZD2, and 36 hours later were subjected to a Matrigel invasion assay. Primary T cells transfected with either WNT5A or FZD2 siRNA demonstrated a diminished migratory response to CXCL12 compared with those transfected with a scrambled siRNA (D); n (donors) = 3, *P < .01. The same is true of a stably transfected WNT5A shRNA cell line (CEM), in which Wnt5A expression is dramatically reduced (E inset) and so is migration in response to CXCL12 (E); *P < .01. These data are supported further by the fact that Rac activation, required for motility of T cells, is increased by rWnt5A (F) in primary T cells and cannot be activated by CXCL12 in WNT5A shRNA-transfected CEM cells (G).
RWnt5A can influence CXCL12-mediated signaling in primary T cells. (A) Western blot analysis demonstrates that phospho-PKC (α, β, and γ isoforms) is up-regulated in primary human T cells by treatment with 0.2 μg/mL rWnt5A for 16 hours. This activation occurs in a biphasic manner. (B) T cells pretreated with rWnt5A for 16 hours demonstrate greater migration in response to CXCL12 in a dose-dependent manner; n (donors) = 3, *P < .01. (C) Western blot analysis demonstrates that in the presence of WNT5A siRNA, 100 ng/mL CXCL12 (12 hours of treatment) failed to increase Wnt5A expression compared with control siRNA-transfected cells. Primary human T cells were then transfected with siRNA against either WNT5A or FZD2, and 36 hours later were subjected to a Matrigel invasion assay. Primary T cells transfected with either WNT5A or FZD2 siRNA demonstrated a diminished migratory response to CXCL12 compared with those transfected with a scrambled siRNA (D); n (donors) = 3, *P < .01. The same is true of a stably transfected WNT5A shRNA cell line (CEM), in which Wnt5A expression is dramatically reduced (E inset) and so is migration in response to CXCL12 (E); *P < .01. These data are supported further by the fact that Rac activation, required for motility of T cells, is increased by rWnt5A (F) in primary T cells and cannot be activated by CXCL12 in WNT5A shRNA-transfected CEM cells (G).
Migration of T cells after CXCL12 activation involves the activation of Rac, small GTP-binding proteins involved in promoting actin polymerization. To determine whether Wnt5A can influence Rac activation, primary human T cells were treated with either CXCL12 or rWnt5A for 1 hour. Both treatments result in the activation of Rac (Figure 2F), but in the presence of WNT5A shRNA, Rac failed to be activated by CXCL12 (Figure 2G), supporting a critical intermediary role for Wnt5A in CXCL12 signaling, and the activation and migration of T cells.
Wnt5A is required for CXCL12 signaling via its effects on CXCR4
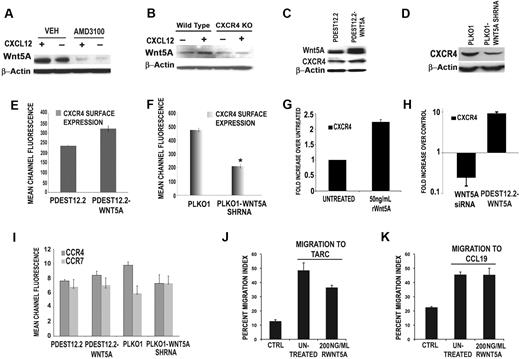
To determine whether the activation of Wnt5A by CXCL12 occurred via CXCR4, T cells were treated with a potent and highly selective antagonist of CXCR4, AMD3100, for 1 hour before the addition of CXCL12 for 12 hours (with continued presence of AMD3100). In the presence of AMD3100, CXCL12 was unable to increase Wnt5A expression (Figure 3A third lane). Interestingly, treatment of T cells with AMD3100 (12 hours) also decreased the expression of Wnt5A itself, beyond baseline levels (Figure 3A fourth lane). Furthermore, T cells isolated from CXCR4-null mutant mice and treated with CXCL12 failed to demonstrate an increase in Wnt5A expression compared with wild-type controls (Figure 3B).
CXCL12 requires CXCR4 to activate Wnt5A, and Wnt5A can modulate CXCR4 expression. Primary human T cells were pretreated with the CXCR4 antagonist, AMD3100 (1 μM, for 1 hour, with continued treatment for 12 hours, in the presence or absence of 100 ng/mL CXCL12). In the presence of AMD3100, CXCL12 is unable to increase Wnt5A expression (A). In CXCR4-null mutant mice, CXCL12 treatment can increase Wnt5A expression in wild-type, but not in CXCR4-null, mice (B). Stable transfection of CEM cells with a plasmid overexpressing WNT5A (pDEST12.2-WNT5A) results in a modest increase (*P < .05) in CXCR4 expression by Western blot analysis (C). Conversely, stable shRNA knockdown of WNT5A (PLKO.1-WNT5A-shRNA) in CEM cells results in a significant decrease in CXCR4 expression (D). This is confirmed by FACS analysis (E-F). RWnt5A treatment (50 ng/mL) of primary T cells resulted in increases in the levels of CXCR4 transcription (G). Transfection of primary T cells with siRNA against WNT5A, resulted in a significant reduction in CXCR4 transcription compared with control siRNA–transfected cells (H). FACS analysis demonstrates that the expression of CCR4 and CCR7, the only other chemokine receptors present on the surface of CEM cells, is not significantly affected by WNT5A overexpression. WNT5A knockdown slightly decreases CCR4, but does not significantly affect CCR7 (I). Migration of CEM cells to either of the ligands for CCR4 and CCR7, that is, TARC (J) and CCL19 (K), is not increased by the addition of rWnt5A, indicating that these effects are specific to the CXCL12/CXCR4 axis.
CXCL12 requires CXCR4 to activate Wnt5A, and Wnt5A can modulate CXCR4 expression. Primary human T cells were pretreated with the CXCR4 antagonist, AMD3100 (1 μM, for 1 hour, with continued treatment for 12 hours, in the presence or absence of 100 ng/mL CXCL12). In the presence of AMD3100, CXCL12 is unable to increase Wnt5A expression (A). In CXCR4-null mutant mice, CXCL12 treatment can increase Wnt5A expression in wild-type, but not in CXCR4-null, mice (B). Stable transfection of CEM cells with a plasmid overexpressing WNT5A (pDEST12.2-WNT5A) results in a modest increase (*P < .05) in CXCR4 expression by Western blot analysis (C). Conversely, stable shRNA knockdown of WNT5A (PLKO.1-WNT5A-shRNA) in CEM cells results in a significant decrease in CXCR4 expression (D). This is confirmed by FACS analysis (E-F). RWnt5A treatment (50 ng/mL) of primary T cells resulted in increases in the levels of CXCR4 transcription (G). Transfection of primary T cells with siRNA against WNT5A, resulted in a significant reduction in CXCR4 transcription compared with control siRNA–transfected cells (H). FACS analysis demonstrates that the expression of CCR4 and CCR7, the only other chemokine receptors present on the surface of CEM cells, is not significantly affected by WNT5A overexpression. WNT5A knockdown slightly decreases CCR4, but does not significantly affect CCR7 (I). Migration of CEM cells to either of the ligands for CCR4 and CCR7, that is, TARC (J) and CCL19 (K), is not increased by the addition of rWnt5A, indicating that these effects are specific to the CXCL12/CXCR4 axis.
To determine whether Wnt5A could influence CXCR4 expression, CEM cells were stably transfected with a plasmid overexpressing the WNT5A gene (pDEST12.2-Wnt5A), and after 24 hours of transfection, both the total and surface levels of CXCR4 protein were examined. CEM cells were also stably transfected with plasmids expressing WNT5A shRNA. WNT5A-transfected cells demonstrated a modest increase in CXCR4 expression compared with empty-vector controls (Figure 3C-E). Knockdown of WNT5A results in significant decrease in CXCR4 expression both by Western (Figure 3D) and FACS analysis (Figure 3F). RWnt5A treatment of primary T cells also resulted in increases in the levels of CXCR4 transcription (Figure 3G), and when primary T cells were treated with siRNA against WNT5A, a significant reduction in CXCR4 transcription was observed compared with control siRNA-transfected cells (Figure 3H). Transfection of primary T cells with pDEST12.2-Wnt5A also increased CXCR4 transcription (Figure 3H).
To determine whether these effects were specific to CXCR4, CEM cells, which also express CCR4 and CCR7, were analyzed for effects on CCR4 and CCR7 expression after WNT5A manipulation. WNT5A overexpression did not significantly affect either of these chemokine receptors (Figure 3I). WNT5A knockdown slightly decreased CCR4, but did not affect CCR7. However, migration to either of the ligands to these receptors, that is, TARC (Figure 3J) and CCL19 (Figure 3K), was not increased by Wnt5A.
CXCL12/CXCR4 and Wnt5A play a role in T-cell migration in vivo
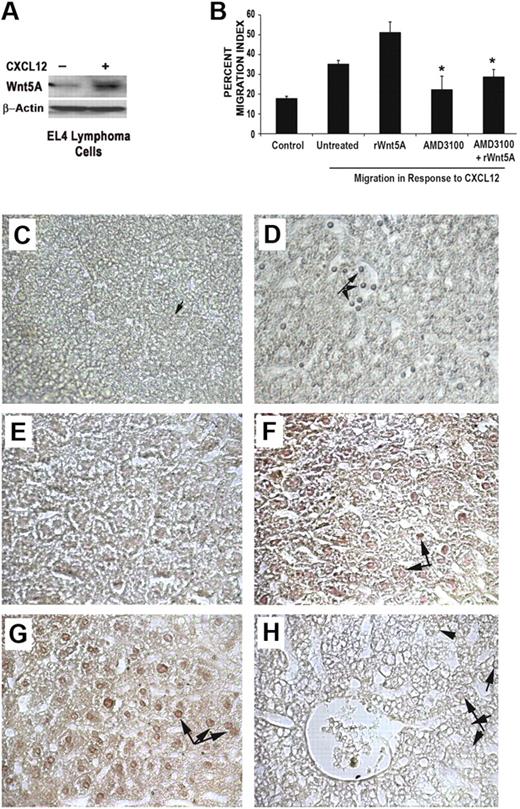
In addition to T-cell signaling, the CXCL12/CXCR4 axis has been shown to play an important role in the motility and metastasis of cancer cells,7 as has Wnt5A.13,16 EL4 cells are derived from a mouse thymoma, and form liver metastases in vivo in C57BL/6 mice.17 To determine whether the CXCL12/CXCR4/Wnt5A axis can play a role in the metastasis of these T-cell tumors, we initially assessed the levels of Wnt5A in these cells by Western blot analysis. EL4 cells constitutively expressed low levels of Wnt5A, and, upon treatment with CXCL12, the Wnt5A levels increased significantly (Figure 4A). Treating EL4 cells with rWnt5A resulted in an increase in CXCL12-induced migration in vitro (Figure 4B), but in the presence of AMD3100 (1 μM), this increase in migration was inhibited. To determine whether Wnt5A and CXCL12 can increase T-cell migration in an in vivo model, in vivo tail vein metastasis assays were used to assay the effects of CXCL12 and Wnt5A on T-cell lymphoma metastasis. Given that EL4 cells are prone to metastasis, we used a lower number of cells (2 × 105) than is common in these assays, as we were focusing on the influence of CXCL12 and Wnt5A on increasing metastasis. EL4 cells were treated overnight either with a vehicle control (PBS with 0.1% bovine serum albumin), CXCL12 (20 ng/mL), or rWnt5A (50 ng/mL), after which the cells were injected via the tail vein, and mice were euthanized after 21 days. During this experiment, PBS, CXCL12, or rWnt5A was injected into the appropriate mice biweekly. After euthanization, livers were extracted, embedded, sectioned, and examined for the presence of EL4 cells using an anti−murine CD3 antibody (Figure 4C-D). In the majority of vehicle control-treated mice, there was minimal infiltration of lymphoma cells into the liver (only 1 of 7 mice showed extensive infiltration). In CXCL12- and Wnt5A-treated mice, there was extensive infiltration into the liver tissue in 5 of 7 and 6 of 7 mice, respectively (Table 1). Where there was extensive EL4 infiltration present in the liver tissue, we observed a decrease in the density of the immediate surrounding tissue (Figure 4D,F-G). In the majority of the liver sections examined in mice treated with CXCL12 (6 of 7) and Wnt5A (7 of 7; Figure 4F-G, respectively, arrows indicate infiltrating EL4 cells), the infiltrating tumor cells (identified by costaining with anti-CD3) were positive for Wnt5A. Interestingly, in control mice, we observed that EL4 cells tended to accumulate and stick to the inside of blood vessels, but these do not appear to be very positive for Wnt5A (data not shown). In the control cells that do infiltrate, there is some positivity for Wnt5A. Similarly, Wnt5A staining in CXCL12-treated EL4 cells was consistently weaker than in Wnt5A-treated cells, albeit higher than in the control cells. Because the sections are stained with both CD3 (alkaline-phospatase development, resulting in a bluish color) and Wnt5A (developed with diaminobenzidine, resulting in a brown color), they are not counterstained with hematoxylin, which would mask the CD3 signal. A section from the liver of a mouse injected with Wnt5A-treated cells, stained only with secondary antibody as a negative control, is shown in Figure 4H. Arrows indicate infiltrating EL4 cells that are difficult to see without staining for CD3 or CD3/Wnt5A. Positive Wnt5A staining correlated to increased infiltration (as determined by costaining with CD3) and a disintegration of the cellular architecture of the liver. Results of Wnt5A staining and effects on liver density are summarized in Table 1. These data imply that CXCL12 and Wnt5A can increase the migration of T cells in vivo, and this increased infiltration correlates with Wnt5A expression.
CXCL12 and Wnt5A can increase T-cell migration in an animal model. EL4 cells treated with CXCL12 (100 ng/mL, 12 hours) demonstrate an increase in Wnt5A expression (A). Treatment of EL4 cells with rWnt5A (50 ng/mL, 16 hours) increases migration in response to CXCL12 (B). AMD3100 (1 μM) inhibits Wnt5A stimulation of CXCL12-induced migration; *P < .01 (B). In vivo data demonstrate that in control animals, after the injection of low numbers of EL4 cells, there are very few metastasizing EL4 cells (C), as demonstrated by CD3 staining, compared with the increased number of CD3-positive EL4 cells in the CXCL12-treated (D arrows) and Wnt5A-treated animals (data not shown). The differences in the density of the livers can be clearly seen, where there are clear spaces surrounding the tumor cells in panel D. Sections from control, CXCL12-, and Wnt5A-treated animals were then costained for Wnt5A and CD3, and there is very little infiltration of Wnt5A+ CD3+ EL4 cells in controls (E) compared with the increased EL4 infiltration observed in the livers from CXCL12 (F)− and Wnt5A (G)−treated mice. (H) The section is from a Wnt5A-treated animal, incubated with no primary antibody, used as a negative control for staining, in which infiltrating cells are still visible (arrows), but do not stain positive for CD3 or Wnt5A. Images were taken at 40× (C-D), and 64× (E-H). No counterstaining with hematoxylin was performed in these sections. Slides were viewed with a Zeiss Axiovert 2000 microscope using 40×/0.60 KorrPh2 with 1.6× amplification and Permount medium (Fisher Scientific). Stains were done as described in “Methods.” Images were acquired using a Zeiss AxioCam color camera (model 412-312) and Axiovision Version 3.1 (Zeiss) image acquisition software. Images were processed using Adobe Photoshop Version 8.0 (Adobe Systems).
CXCL12 and Wnt5A can increase T-cell migration in an animal model. EL4 cells treated with CXCL12 (100 ng/mL, 12 hours) demonstrate an increase in Wnt5A expression (A). Treatment of EL4 cells with rWnt5A (50 ng/mL, 16 hours) increases migration in response to CXCL12 (B). AMD3100 (1 μM) inhibits Wnt5A stimulation of CXCL12-induced migration; *P < .01 (B). In vivo data demonstrate that in control animals, after the injection of low numbers of EL4 cells, there are very few metastasizing EL4 cells (C), as demonstrated by CD3 staining, compared with the increased number of CD3-positive EL4 cells in the CXCL12-treated (D arrows) and Wnt5A-treated animals (data not shown). The differences in the density of the livers can be clearly seen, where there are clear spaces surrounding the tumor cells in panel D. Sections from control, CXCL12-, and Wnt5A-treated animals were then costained for Wnt5A and CD3, and there is very little infiltration of Wnt5A+ CD3+ EL4 cells in controls (E) compared with the increased EL4 infiltration observed in the livers from CXCL12 (F)− and Wnt5A (G)−treated mice. (H) The section is from a Wnt5A-treated animal, incubated with no primary antibody, used as a negative control for staining, in which infiltrating cells are still visible (arrows), but do not stain positive for CD3 or Wnt5A. Images were taken at 40× (C-D), and 64× (E-H). No counterstaining with hematoxylin was performed in these sections. Slides were viewed with a Zeiss Axiovert 2000 microscope using 40×/0.60 KorrPh2 with 1.6× amplification and Permount medium (Fisher Scientific). Stains were done as described in “Methods.” Images were acquired using a Zeiss AxioCam color camera (model 412-312) and Axiovision Version 3.1 (Zeiss) image acquisition software. Images were processed using Adobe Photoshop Version 8.0 (Adobe Systems).
Summary of EL4 liver metastasis in CXCL12- and Wnt5A-treated mice
| Mouse group . | High infiltration . | Liver density . | Wnt5A staining . | ||||
|---|---|---|---|---|---|---|---|
| Dense . | Weakly affected . | Affected . | Negative . | Weakly positive . | Positive . | ||
| Control | 1/7 | 4* | 2† | 1 | 4* | 2† | 1 |
| CXCL12 | 5/7 | 1* | 2† | 4 | 1* | 2† | 4 |
| Wnt5A | 6/7 | 0 | 1† | 6 | 0 | 1† | 6 |
| Mouse group . | High infiltration . | Liver density . | Wnt5A staining . | ||||
|---|---|---|---|---|---|---|---|
| Dense . | Weakly affected . | Affected . | Negative . | Weakly positive . | Positive . | ||
| Control | 1/7 | 4* | 2† | 1 | 4* | 2† | 1 |
| CXCL12 | 5/7 | 1* | 2† | 4 | 1* | 2† | 4 |
| Wnt5A | 6/7 | 0 | 1† | 6 | 0 | 1† | 6 |
EL4 cells infiltrate extensively into the liver in 5 of 7 and 6 of 7 CXCL12 and Wnt5A-treated animals, respectively. In control animals, only 1 of 7 demonstrate extensive EL4 infiltration into the liver, and in the 1 animal with high infiltration, EL4 cells are positive for Wnt5A. In livers with a high amount of infiltration, the liver architecture is severely compromised, appearing less dense, and Wnt5A staining is intense in the infiltrating EL4 cells.
Animals with no liver infiltration, in which the liver architecture remains dense.
Animals with low levels of infiltration, in which Wnt5A staining is quite weak in the infiltrating EL4 cells, and the liver architecture is only slightly affected.
Discussion
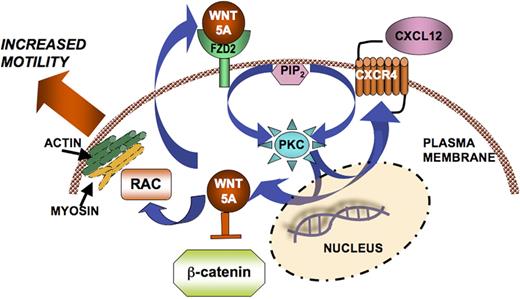
In our current study, we demonstrate that CXCL12-CXCR4 interactions induce the expression and the activation of an intermediate G protein−coupled receptor, Fzd2, and its ligand, Wnt5A. This interaction appears to be specific for this chemokine ligand-receptor pair, as we failed to observe any effects of CCL19 on Wnt5A expression in these cells (Figure 1F). We also demonstrate that T cells express basal levels of Wnt5A. We show that Wnt5A is important for the maintenance of CXCR4 expression, and Wnt5A does not affect other chemokine receptors such as CCR4 and CCR7. Taken as a whole, our data point to a complex regulatory system, in which Wnt5A is required for CXCR4 activity, and in the presence of CXCL12, Wnt5A expression is increased, presumably via increases in PKC. Wnt5A expression in turn can increase the expression of PKC and CXCR4, resulting in a positive feedback loop that has been described in other cell systems.16 We hypothesize that this loop may ultimately act to increase T-cell motility by the mobilization of rac, actin, and myosin, as has been shown for melanoma cells12 (Figure 5). It is interesting that the activity and not simply expression of CXCR4 is critical for the maintenance of Wnt5A expression, suggestive of an even more complex network that we do not clearly understand.
Schematic of Wnt5A signaling in CXC12-mediated T-cell migration. CXCL12 binds to CXCR4 and activates PKC. Both PKC activation and CXCL12/CXCR4 signaling, possibly via unknown mechanisms, can increase the transcription and translation of Wnt5A. This can act in a positive feedback loop, in which Wnt5A can then increase CXCR4 expression, resulting in increased signaling, and ultimately increased migration, perhaps via Rac, actin, and myosin, as demonstrated by Witze et al.12
Schematic of Wnt5A signaling in CXC12-mediated T-cell migration. CXCL12 binds to CXCR4 and activates PKC. Both PKC activation and CXCL12/CXCR4 signaling, possibly via unknown mechanisms, can increase the transcription and translation of Wnt5A. This can act in a positive feedback loop, in which Wnt5A can then increase CXCR4 expression, resulting in increased signaling, and ultimately increased migration, perhaps via Rac, actin, and myosin, as demonstrated by Witze et al.12
Although there are few data on the interplay between the Wnts, CXCL12, and CXCR4, a recent report by Luo et al18 reported that, in neuronal progenitors, the canonical Wnt pathway is a target of CXCL12 signaling. In contrast, Tickenbrock et al19 have demonstrated that activation of the canonical Wnt pathway in human monocytes using Wnt3A actually results in a decreased migration rate through an endothelial monolayer due to an augmentation in their adhesion to endothelial cells. In our current report, we have demonstrated that CXCL12 stimulation of human T cells directly induces the transcription and expression of Wnt5A and Fzd2, which interact to facilitate CXCL12-mediated migration and in maintaining the lymphocyte expression of CXCR4. This Wnt5A-dependent pathway appears to be distinct from the β-catenin–driven, promigratory effects on T cells described by Wu et al,11 in that β-catenin is actually down-regulated by CXCL12 treatment and shRNA knockdown of β-catenin results in no effect on the CXCL12-mediated migration of human T cells (data not shown). Our data indicate that CXCL12-stimulated T cells demonstrate enhanced expression of various members of the noncanonical Wnt pathway (eg, Fzd2, Wnt5A), while concurrently suppressing those of the canonical/β-catenin pathway (eg, β-catenin, Wnt3A). This observation is in keeping with data that show that Wnt5A promotes the degradation of β-catenin via a Siah2/adenomatous polyposis coli–dependent pathway.20 However, it is still possible that canonical Wnt signaling may play a role in T-cell motility, which requires more experimentation to accurately decipher.
The role of Wnt5A in the migration, as opposed to proliferation, of T-cell cancers is unclear. It has been shown that in these cancers, Wnt5A can act as a tumor suppressor, inhibiting the proliferation of T-cell tumors.21,22 T-cell leukemias tend toward an increase in Wnt1/β-catenin/lymphoid enhancer factor/T-cell–specific factor (TCF) signaling, and a loss of Wnt5A.23 However, this is not the case for all T-cell tumors. It has recently been shown that peripheral T lymphomas may be of either T helper cell (Th) 1 or Th2 origin, and although Th1 lymphomas are strongly positive for TCF, a downstream effector of β-catenin, those of Th2 origin were, without exception, negative for TCF/lymphoid enhancer factor.24 Because Wnt5A is an inhibitor of canonical Wnt signaling, it may be that in these tumor types, Wnt5A is up-regulated. In EL4 cells, it has been shown that metastasis of tumors in EL4-bearing mice can be induced by treatment of transforming growth factor β, and that this treatment also shifts cells from a Th1- to a more Th2-dominant phenotype.25 This shift can also occur upon treatment with phorbol myristate acetate,25 and given that Wnt5A can activate PKC, it is possible that Wnt5A treatment may also push EL4 cells to the more aggressive Th2 phenotype, linked to the suppression of β-catenin signaling.
In summary, we show in this study, for the first time, that Wnt5A expression is up-regulated by CXCL12, and, moreover, is a critical intermediate of CXCL12/CXCR4 signaling. This requirement is unique in that there are no published studies demonstrating the ability of a chemokine to induce the transcription and translation of specific members of the noncanonical Wnt signaling pathway. In addition, Wnt5A modulation of CXCR4 activity and expression may have important implications for cell trafficking and engraftment, as well as in diseases such as AIDS, in which HIV entry involves chemokine coreceptors such as CXCR4.26 It is clear that the role of Wnt5A in chemokine biology and in diseases of immune origin requires much more attention and elucidation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institute on Aging, National Institutes of Health.
National Institutes of Health
Authorship
Contribution: M.C.G., G.D.C., B.V., K.P., M.B., A.C., A.L., K.G.B., W.W.W., C.D.E., A.D.F., M.P.O., M.X., and A.T.W. performed the experiments; M.C.G., A.T.W., and D.D.T. wrote the paper; and A.T.W. and D.D.T. designed the research.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ashani T. Weeraratna or Dennis D. Taub, Laboratory of Immunology, National Institute on Aging, National Institutes of Health, Gerontology Research Center, 5600 Nathan Shock Dr, Baltimore, MD 21224; e-mail: weerarat@grc.nia.nih.gov or taubd@grc.nia.nih.gov.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal