Abstract
Blood vessel maturation and stability require recruitment of mural cells (MCs) to the nascent vessel. Loss or detachment of MCs causes vascular dysfunction in diseases. N-sulfation of heparan sulfate (HS) is required for platelet-derived growth factor B (PDGF-B) retention and platelet-derived growth factor receptor-β (PDGFR-β) signaling during MC recruitment. To analyze the specific role of MC-derived HS in this process, we inactivated HS synthesis in MCs. MC-specific loss of HS causes embryonic lethality associated with vascular patterning defects, edema, and hemorrhages during late gestation. MC recruitment in the skin is impaired, correlating with defective PDGFR-β and transforming growth factor-β (TGF-β)–SMAD signaling. Accumulation of rounded cells positive for MC markers close to the vessels indicates defective polarization and migration of local MC progenitors. In contrast, MC recruitment and signaling in the central nervous system (CNS) are unaffected by MC HS loss. Our results suggest that HS is selectively required in a cell-autonomous manner, acting in cis with PDGFR-β and TGF-β receptors during induction/polarization and migration of local progenitor cells to the nascent vessel. Once MCs are in contact with the vessel, as during CNS vascularization, endothelial HS appears sufficient to facilitate PDGFR-β activation in trans.
Introduction
Tumor blood vessels have multiple structural and functional abnormalities. A characteristic of the tumor vasculature is the deficiency or loose attachment of mural cells (MCs), that is, vascular smooth muscle cells in arteries, arterioles, and veins and pericytes (PCs) in capillaries. In many pathologic processes, such as diabetic retinopathy, the absence and loss of PC coverage cause destabilization and subsequent regression of blood vessels as well as increased permeability resulting in an abnormal and leaky vasculature.1,2
MCs are key players in vessel stabilization and maturation. MCs may contribute to the stability of the vessel by providing direct mechanical support, through matrix deposition and/or by the release, presentation and activation of signals that promote endothelial cell (EC) differentiation and quiescence.3
Several ligand-receptor systems have been implicated in this process. Transforming growth factor-β (TGF-β) regulates proliferation and differentiation of ECs and MCs4 and mediates the de novo induction of MC from the mesenchymal cell lineage during embryonic development.5 Ex vivo, TGF-β becomes active on close cell-cell contact, via gap junctions between MCs and ECs.4 Recent studies also illustrate that components of the TGF-β pathway, including TGF-β receptors, interact and cocluster directly with VE-cadherin at EC-EC junctions, thereby promoting vessel stabilization and quiescence.6 In addition, junction organization leads to changes in cell function, and cell-cell-contact regulates many genes implicated in cell growth, apoptosis, matrix, and cytoskeletal remodeling.7
Detailed studies on mouse mutants demonstrated the fundamental importance of endothelial platelet-derived growth factor B (PDGF-B) signaling to PDGF receptor-β (PDGFR-β) on MCs during the recruitment process.8,9 Deletion of Pdgfb or Pdgfrb causes embryonic lethality resulting from vascular defects, including microaneurysms, vascular leakage, and hemorrhaging as a result of severe MC loss.9 Conditional endothelial inactivation of Pdgfb illustrated that the endothelium provides the major source of PDGF-B required for migration and proliferation of MCs. PDGF-B protein is secreted by the endothelium as PDGF-BB homodimers. PDGF-BB binding to PDGFR-β on MCs leads to receptor dimerization and phosphorylation activating multiple downstream signaling pathways, including phosphoinositide 3-kinase and extracellular regulatory kinase 1/2 (Erk1/2) mitogen-activated protein kinase (MAPK), which stimulate migration and proliferation.
PDGF-B contains a conserved C-terminal sequence of basic amino acids, a so-called retention motif that mediates binding to cell surface or extracellular matrix (ECM) heparan sulfate proteoglycan (HSPG). Mice deficient in the PDGF-B retention motif display vascular defects associated with PC detachment. Detailed studies on developmental and tumor angiogenesis led to the idea that heparan sulfate (HS) retains PDGF-BB close to the EC surface to facilitate directed MC migration along the sprouting vessel and to mediate proper MC attachment to the vessel wall.10,11
We recently showed that altered HS biosynthesis producing a general reduction of N-sulfation on the HS chain leads to MC detachment and delayed MC migration. Appropriate N-sulfation is required to retain PDGF-BB and to activate PDGFR-β in MCs in vivo.1,12 Therefore, HSPGs are important coreceptors and activators of signaling events during MC recruitment.
Whether MC-derived HS is required for vessel formation and stabilization in vivo remains unclear, and we currently lack all information on the cell-specific and cell-autonomous requirement of HS during developmental angiogenesis. Here we address the relevance of MC-derived HS for MC recruitment and attachment in mice by selective genetic deletion of the glycosyltransferase Ext1 in the MC population. We find that MC HS is required for induction and recruitment of mesenchymal MC progenitors in the periphery, whereas MCs that are in contact with the endothelium during brain angiogenesis can be recruited independently of HS production. Our results suggest that the spatial proximity of signal sending cells (the ECs) and receiving cell (MCs and progenitors) determines whether or not the receiving cell requires cell-autonomous HS production to support receptor activation.
Methods
Animals
Animals were housed in individually ventilated cages under barrier conditions in the animal facility of the London Research Institute (London, United Kingdom). Pdgfrb-Cre13 and EXT1flox/flox mice14 were bred to generate Pdgfrb Cre EXT1flox/flox mice, referred to as EXT1MCko in C57Bl/6 background. Cre-negative littermates were used as controls. Cre activity of transgenic founders was characterized in a ROSA26R EYFP Cre reporter background.15
All procedures were performed in accordance with United Kingdom Home Office regulations and approved by the local animal ethics committee at London Research Institute.
Immunhistochemistry
Embryos were isolated between embryonic day (E)10.5 and E19.5 postcoitum and fixed in 4% paraformaldehyde for 2 to 16 hours. Tail and yolk sac biopsies were taken to determine the genotype by polymerase chain reaction (PCR) using DNA prepared from biopsies (as previously described13,14 ).
Hindbrains were dissected clean from other tissues and whole-mount stained as previously described.16,17 Blood vessels were detected using biotinylated Griffonia simplicifolia isolectin-B4 (20 μg/mL, Sigma-Aldrich), and PCs were labeled using rabbit anti-NG2 antibody (1:200; Chemicon International). HS was detected using 10E4 antibody (1:100; Seikagaku America). Secondary antibodies conjugated with the appropriate fluorochrome, AlexaFluor 488, 568, or 633 (Invitrogen) were used. Hindbrains were mounted on glass slides with Mowiol supplemented with antibleaching agent 1,4-diazabicyclo-2.2.2-octane (Sigma-Aldrich). Mounted hindbrains and sections were analyzed by conventional light microscopy and fluorescence microscopy using Zeiss Axiovert 200 equipped with a digital camera (Carl Zeiss) and by confocal laser scanning microscopy using a Zeiss LSM Meta 510. Digital images were processed using Volocity (Improvision) and Adobe Photoshop CS2.
Whole-mount staining of E13.5 embryos was performed after methanol/PBT (phosphate-buffered saline [PBS] containing 0.1% Tween 20) fixation and stepwise rehydration. Samples were passed through methanol/PBT gradient 50%, 80%, 100%, 80%, 50%, PBT. After permeabilization with PBlec (PBS-A, pH 6.8, containing 1% Triton X-100, 0.1 mM CaCl2, 0.1 mM MgCl2, 0.1 mM MnCl2), blood vessels were detected using a hybridoma supernatant recognizing endomucin (kind gift of D. Vestweber, Max Planck Institute for Molecular Biomedicine, Muenster, Germany) diluted 1:20 in PBlec. Pericytes were colabeled using rabbit anti-NG2 (1:200 in PBlec, Chemicon International) and rabbit anti–collagen IV (CIV, 1:100, Biogenesis) was used to detect basement membrane (BM) of regressing vessels.
Regression was determined by measuring the length of CIV-positive but endomucin-negative profiles using the line measurement tool of Volocity 4.3.1 (Improvision software). Quantification and graphic illustration represent a comparison of the sum of regression profile length per image analyzed. Total vessel length per image was quantified measuring the skeletal length of endomucin-positive vessels.
Vessel diameter was quantified measuring the width of vessel profiles using the line measurement tool of Volocity (Improvision).
Coronary vessels of embryonic heart were visualized using purified anti–mouse CD31 (PECAM-1, BD Biosciences PharMingen).
Antibodies used for immunohistochemistry on paraffin sections of E13.5 and E16.5 embryos were as follows: TdT-mediated dUTP nick end labeling (TUNEL) assay (Promega), polyclonal rabbit anti–human caspase 3 active antibody (1:750, R&D Systems), polyclonal rabbit anti–human phospho-histone H3 (1:250, Upstate Biotechnology), endomucin NG2 (1:100, Chemicon International), rabbit anti–LYVE-1 (1:1000, RELIA Tech), 10E4 (1:50, Seikagaku America), and pSMAD3 (1:100, Epitomics).
Secondary antibodies conjugated with the appropriate fluorochrome, AlexaFluor 488 or 568 (Invitrogen) were used and analyzed using confocal laser scanning microscopy using a Zeiss LSM Meta 510 (Carl Zeiss). Samples were viewed with a 63×/1.2 numeric aperture (NA) water-immersion C-APOCHROMAT, 40×/1.2 NA water-immersion C-APOCHROMAT, 25×/0.8 NA water-immersion Plan-NEOFLUAR, and 10×/0.45 NA water-immersion and Mowiol Mount medium containing antibleaching agent. Images were analyzed using Volocity 4.3.1 (Improvision) and assembled using Adobe Photoshop CS2 (Adobe Systems). Overview images of embryonic vasculature were viewed using StemiSV11 and acquired using AxioCam HRc (Carl Zeiss).
Signaling assays
Embryos were isolated at E13.5, skin samples of the embryonic back displaying vascular defects and edema were immediately dissected in ice-cold PBS and snap-frozen on dry ice.
Whole protein lysates were obtained applying radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris-HCl, pH 8.0, 150 mM NaCl2, 1% NP-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate) containing ethylenediaminetetraacetic acid-free protease inhibitor (1 tablet Complete Mini/0 mL RIPA buffer, Roche Diagnostics) and phosphatase inhibitor cocktail (1:100, Sigma-Aldrich). Samples were homogenized using microprobe and sonicated additionally for 10 seconds. Protein lysates were centrifuged for 10 minutes at 10 000g, 4°C.
To detect phosphoVEGFR2 levels, protein lysates of entire embryos (E13.5) were immunoprecipitated using VEGFR2 antibody (Cell Signaling Technology). Antibody was added to the lysate at a final concentration of 1.5 μg/mL and incubated for 1.5 hours on ice. Protein A-Sepharose beads were washed in RIPA buffer, and slurry was added 1:1 to the protein-antibody-lysate and incubated for 45 minutes at 4°C. Samples were centrifuged at 3000g, supernatant was removed, and Sepharose was washed with RIPA buffer. Sample loading buffer was added, heated for 7 minutes at 95°C, and supernatant was loaded on the gel.
Reducing sodium dodecyl sulfate sample buffer was added, and samples were boiled for 10 minutes at 70°C. Cell extracts were separated on NuPAGE 4% to 12% Bis-Tris gel (Invitrogen) and transferred to a Hybond-P polyvinylidene difluoride membrane (GE Healthcare) according to the manufacturer's instructions. The membrane was incubated with the antibodies provided in the PathScan PDGFR Tyrosine Kinase Activity Assay Kit (7180; Cell Signaling Technology) or antibodies directed against phosphoVEGFR2 and VEGFR2 (Cell Signaling Technology), pSMAD3 (1:1000; Epitomics), pSMAD2 (1:1000; Chemicon International), and β-tubulin (1:3000; Covance Research Products). Βeta-tubulin served as a loading control. Secondary antibodies conjugated with enhanced chemiluminescence anti–rabbit and anti–mouse (1:10 000, GE Healthcare) were applied to detect protein expression. The membranes were developed using LumiGLO provided with the PDGFR Tyrosine Kinase Activity kit or detection reagents from GE Healthcare, respectively.
Statistical analysis
Statistical analysis was performed with Prism 5.0a software (GraphPad Software) using 2-tailed, unpaired t test and F, showing variances. P value less than .05 was considered statistically significant.
Results
Genetic approach for the deletion of HS in MCs
The Ext1 gene encodes for the key glycosyltransferase that is essential for elongation of the disaccharide backbone chain of HS. The EXT1 protein forms homodimers or heterodimers with EXT2 and resides as single-pass transmembrane protein in the Golgi apparatus. Deletion of Ext1 leads to complete loss of HS. The original observation that loss-of-function mutations cause multiple exostosis in humans led to the name Exostosis protein (EXT1). To study the functional role of MC-derived HS in angiogenesis, we deleted the floxed EXT1 allele14 selectively in MCs using transgenic Pdgrfb Cre mice.13 This genetic approach results in the excision of EXT1 in MCs (for simplicity, we refer to Pdgfrb Cre+/Ext1flox/flox mice as EXT1MCko).
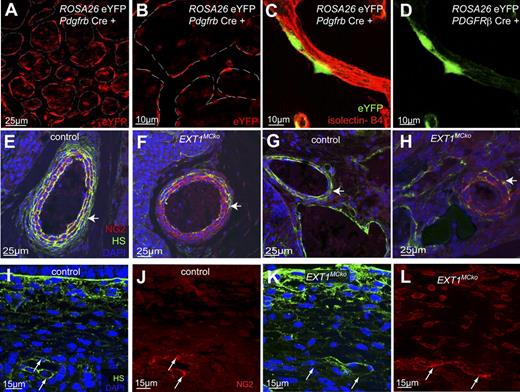
We initially confirmed the selectivity of Cre-mediated deletion in MCs at embryonic stages by crossing the Pdgfrb Cre to the ROSA26R EYFP reporter line.15 Analysis of embryonic skin whole mounts at E13.5 (Figure 1A-B) and E17.5 (data not shown) reveals nuclear and cytoplasmic eYFP staining in MCs closely attached to blood vessels (dashed line). The eYFP expression pattern (Figure 1A-B) closely resembles MC staining, confirming previous reports of specific and efficient Cre-mediated recombination in MC in skin samples.13,18 Isolated eYFP expression in cells not directly abutted to vessel profiles suggests that mesenchymal cells in the vicinity of blood vessels also undergo Cre recombination driven by the pdgfrb promoter. ECs, however, never showed eYFP signal. In the hindbrain, double labeling of eYFP and ECs with isolectin B4 confirmed specific Cre recombination in PCs, but not ECs (Figure 1C-D). Unlike in skin samples, all eYFP signal observed in hindbrain samples is consistently associated with vessel profiles. This is in agreement with PC-specific expression of pdgfrb and the absence of isolated mesenchymal PC progenitors during brain angiogenesis.
MC-specific deletion of HS.Pdgfrb Cre line specificity detected by ROSA26 eYFP expression (A) and (B) in embryonic skin (n = 2). Colabeling of embryonic hindbrains of Pdgfrb Cre ROSA26 EYFP at E10.5 with isolectin B4 (red, C) revealed PC-specific expression of eYFP (green, C,D); n = 4. Sections of E13.5 embryos were stained with an antibody directed against the HS chain (green), NG2 (red), and 4,6-diamidino-2-phenylindole. Vascular smooth muscle cells (vSMCs) of the dorsal aorta (E,F) have strongly reduced HS deposition with residual HS on the outermost layer of vSMCs in EXT1MCko embryos (arrow, F). Immunostaining revealed loss of HS in vSMCs and PCs of EXT1MCko (F,H), whereas HS is strongly expressed in control littermates (E,G) and localized at the abluminal surface of MCs. High magnification imaging of capillaries in the embryonic skin (I-L) showed colocalization of HS and NG2 in control sections (arrows, I.J), whereas NG2-positive cells in EXT1MCko sections lack HS (arrows, K,L).
MC-specific deletion of HS.Pdgfrb Cre line specificity detected by ROSA26 eYFP expression (A) and (B) in embryonic skin (n = 2). Colabeling of embryonic hindbrains of Pdgfrb Cre ROSA26 EYFP at E10.5 with isolectin B4 (red, C) revealed PC-specific expression of eYFP (green, C,D); n = 4. Sections of E13.5 embryos were stained with an antibody directed against the HS chain (green), NG2 (red), and 4,6-diamidino-2-phenylindole. Vascular smooth muscle cells (vSMCs) of the dorsal aorta (E,F) have strongly reduced HS deposition with residual HS on the outermost layer of vSMCs in EXT1MCko embryos (arrow, F). Immunostaining revealed loss of HS in vSMCs and PCs of EXT1MCko (F,H), whereas HS is strongly expressed in control littermates (E,G) and localized at the abluminal surface of MCs. High magnification imaging of capillaries in the embryonic skin (I-L) showed colocalization of HS and NG2 in control sections (arrows, I.J), whereas NG2-positive cells in EXT1MCko sections lack HS (arrows, K,L).
HS staining on paraffin sections confirms substantial loss of HS in the MC compartment of major arteries and veins of EXT1MCko embryos (Figure 1E-H). In addition, smaller vessels consistently reveal loss of HS staining in MCs, whereas endothelial HS staining persists (Figure 1I-L). The 10E4 antibody is widely used to detect HS and recognizes an epitope that is destroyed by N-desulfation.19 Colabeling of HS (10E4) and MCs using NG2 antibodies that selectively label MCs in many tissues20 indicates that a small fraction of MCs does not recombine the floxed EXT1 allele (Figure 1F,H arrows). Alternatively, slow HS turnover and more recent recruitment could explain residual HS as more cells of the outermost smooth muscle layer around arteries retain HS staining (Figure 1F). Effective depletion of HS from most MCs allows us to address, for the first time, the functional importance of MC HS expression for vascular development.
MC HS is not required for MC recruitment in the CNS
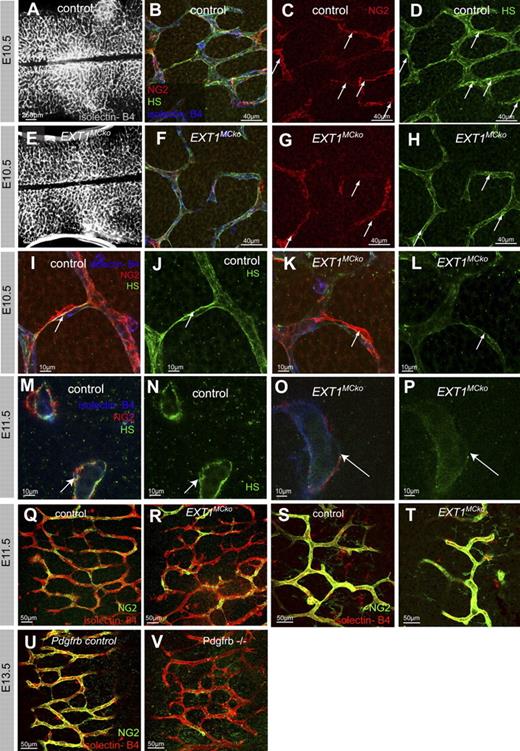
EXT1MCko embryos harvested at E10.5 are macroscopically indistinguishable from control littermates. We labeled hindbrain samples with isolectin-B4 to visualize ECs and NG2 antibody to detect MCs (Figure 2). Overview images of the developing vessel network in the hindbrain show no significant differences in vessel formation, patterning, and vessel density (Figure 2A,E).
Blood vessel formation and MC recruitment in the CNS do not require HS production by MCs. Overview images of the vasculature from control (A) and EXT1MCko (E) hindbrains. Whole-mount hindbrains of E11.5 embryos were labeled with isolectin-B4 (blue) and NG2 (red) to detect ECs and MCs, respectively. High-resolution imaging revealed recruitment and attachment of MCs in EXT1MCko (F) as well as control (B) embryos. Immunostaining with HS (green) confirmed the presence of HS in BM of blood vessels (arrows, D,H); n > 5. Arrows (C-D,G-H) highlight strongest HS expression correlating with MC association to vessels. High-magnification microscopy revealed solid HS expression at the EC-MC interface in vessels of control embryos (arrow, I-J,M-N), lacking in EXT1MCko vessels (K-L,O-P). Overall vascular plexus in EXT1MCko hindbrains displays reduced HS expression (L,P); n > 4. Comparison of MC coverage in EXT1MCko and control hindbrains at the periphery (Q-R) and midline (S-T) shows no differences in MC recruitment and migration; n = 3. In contrast, Pdgfrb−/− hindbrains (V) lack MC coverage compared with control littermates (U); n = 2.
Blood vessel formation and MC recruitment in the CNS do not require HS production by MCs. Overview images of the vasculature from control (A) and EXT1MCko (E) hindbrains. Whole-mount hindbrains of E11.5 embryos were labeled with isolectin-B4 (blue) and NG2 (red) to detect ECs and MCs, respectively. High-resolution imaging revealed recruitment and attachment of MCs in EXT1MCko (F) as well as control (B) embryos. Immunostaining with HS (green) confirmed the presence of HS in BM of blood vessels (arrows, D,H); n > 5. Arrows (C-D,G-H) highlight strongest HS expression correlating with MC association to vessels. High-magnification microscopy revealed solid HS expression at the EC-MC interface in vessels of control embryos (arrow, I-J,M-N), lacking in EXT1MCko vessels (K-L,O-P). Overall vascular plexus in EXT1MCko hindbrains displays reduced HS expression (L,P); n > 4. Comparison of MC coverage in EXT1MCko and control hindbrains at the periphery (Q-R) and midline (S-T) shows no differences in MC recruitment and migration; n = 3. In contrast, Pdgfrb−/− hindbrains (V) lack MC coverage compared with control littermates (U); n = 2.
Confocal microscopy analysis of EXT1MCko hindbrains reveals that MCs are recruited to the blood vessels and closely associated with the endothelium, as seen in control littermates (Figure 2B,F,I,K,M,O). Detailed analysis of MC investment at the midline and periphery of hindbrains shows no defects in MC recruitment (Figure 2Q-T). This lack of phenotype stands in marked contrast to findings in mice deficient in PDGFR-β signaling, such as Pdgfrb−/− (Figure 2V), the PDGF-B retention motif knockout,11 and the Ndst1−/− mice with a global reduction in N-sulfated HS.1,12 Both the PDGF-B retention motif knockout and the Ndst1−/− mice show defective PC recruitment resulting from absent or reduced ability of PDGF-BB to bind to HS.1,12
To address whether the attached MCs in the EXT1MCko indeed lack HS expression, we labeled the hindbrains with the 10E4 antibody (Figure 2B,D,F,H-P). In control hindbrain samples, we detect HS in the endothelium as well as in MCs, particularly at the interface of ECs and MCs (Figure 2C,D,I,M arrows). In contrast, no HS decorates the membrane of MCs in the mutant and significantly less HS is found at the abluminal side of the endothelium of mutant vessels (Figure 2G-H,L,P arrows), suggesting that pericytic HS is indeed absent and normally contributes to the BM surrounding the vessel. These results indicate, however, that pericytic HS production is not required for MC recruitment during sprouting angiogenesis in the developing central nervous system (CNS).
Lack of MC HS results in abnormal vessel morphology and reduced vessel stability
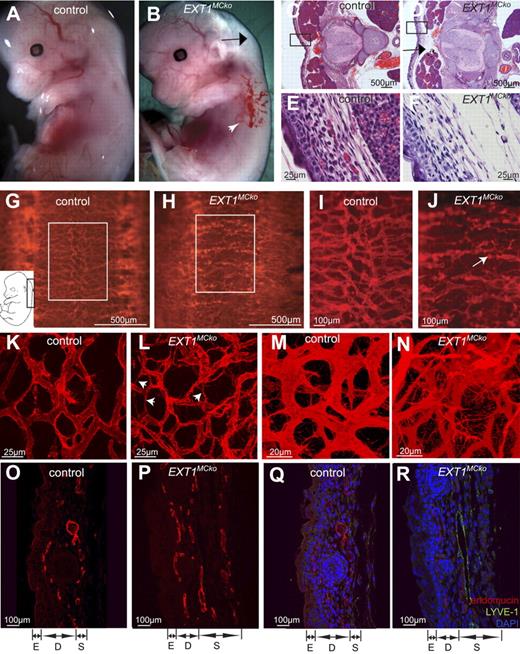
Despite apparently normal vascular patterning at E11.5, no viable mutants were born, indicating that the deletion of pericytic HS causes embryonic lethality at later stages. Further analysis revealed that lethality occurs between E13.5 and E19. At E13.5, EXT1MCko embryos exhibit hemorrhages (Figure 3B white arrowhead) and edema (Figure 3B black arrow) in the skin. Histologic transverse sections illustrate edema formation visible as increased interstitial space between the skin, spinal cord, and muscle blocks (Figure 3C,D arrow). Loose connective tissue in the hypodermis of mutant embryos further confirms the presence of fluid accumulation (Figure 3E-F). However, erythrocyte extravasation is only occasionally observed, implicating increased plasma leakage and/or poor lymphatic uptake as primary cause of edema formation.
Abnormal vessel morphology and patterning defects in EXT1MCko. Macroscopic analysis of E13.5 control (A) and EXT1MCKO embryos revealed edema ( , B) and hemorrhages (white arrowhead, B) in the skin; n > 3. Transverse sections demonstrate increased interstitial space (D,
, B) and hemorrhages (white arrowhead, B) in the skin; n > 3. Transverse sections demonstrate increased interstitial space (D,  ) and loose connective tissue in the epidermis of EXT1MCko (F) compared with the control littermate (C,E); n = 2. Endomucin staining (G-N, red) of E13.5 mutant embryos revealed abnormal patterning of the superficial vessels at the back along the spinal cord (G-H). (I-J) Higher-magnification images of the boxed area in panels G and H. The skin vasculature of the control littermates is uniform in its size and diameter (K). In contrast, EXT1MCko display irregular-shaped, tortuous vessels with fluctuation in their diameter (arrowheads, L). EXT1MCko exhibit excessive filopodia extension (N), indicating aberrant sprouting processes, which are reflected in embryonic skin sections showing a widespread network of blood vessels through the entire depth of the skin (P). On the contrary, the skin vasculature in control embryos is restricted to a two-dimensional network (M,O); n > 5. Visualization of lymphatic vessels (LYVE-1, green) revealed an increase in lymphatic vessels in EXT1MCko embryonic skin (R) compared with control littermates (Q); however, colabeling with endomucin (red) showed separation of lymphatic and blood vessels and no defects in lymphangiogenesis. E indicates epidermis; D, dermis; S, subcutis. n = 2.
) and loose connective tissue in the epidermis of EXT1MCko (F) compared with the control littermate (C,E); n = 2. Endomucin staining (G-N, red) of E13.5 mutant embryos revealed abnormal patterning of the superficial vessels at the back along the spinal cord (G-H). (I-J) Higher-magnification images of the boxed area in panels G and H. The skin vasculature of the control littermates is uniform in its size and diameter (K). In contrast, EXT1MCko display irregular-shaped, tortuous vessels with fluctuation in their diameter (arrowheads, L). EXT1MCko exhibit excessive filopodia extension (N), indicating aberrant sprouting processes, which are reflected in embryonic skin sections showing a widespread network of blood vessels through the entire depth of the skin (P). On the contrary, the skin vasculature in control embryos is restricted to a two-dimensional network (M,O); n > 5. Visualization of lymphatic vessels (LYVE-1, green) revealed an increase in lymphatic vessels in EXT1MCko embryonic skin (R) compared with control littermates (Q); however, colabeling with endomucin (red) showed separation of lymphatic and blood vessels and no defects in lymphangiogenesis. E indicates epidermis; D, dermis; S, subcutis. n = 2.
Abnormal vessel morphology and patterning defects in EXT1MCko. Macroscopic analysis of E13.5 control (A) and EXT1MCKO embryos revealed edema ( , B) and hemorrhages (white arrowhead, B) in the skin; n > 3. Transverse sections demonstrate increased interstitial space (D,
, B) and hemorrhages (white arrowhead, B) in the skin; n > 3. Transverse sections demonstrate increased interstitial space (D,  ) and loose connective tissue in the epidermis of EXT1MCko (F) compared with the control littermate (C,E); n = 2. Endomucin staining (G-N, red) of E13.5 mutant embryos revealed abnormal patterning of the superficial vessels at the back along the spinal cord (G-H). (I-J) Higher-magnification images of the boxed area in panels G and H. The skin vasculature of the control littermates is uniform in its size and diameter (K). In contrast, EXT1MCko display irregular-shaped, tortuous vessels with fluctuation in their diameter (arrowheads, L). EXT1MCko exhibit excessive filopodia extension (N), indicating aberrant sprouting processes, which are reflected in embryonic skin sections showing a widespread network of blood vessels through the entire depth of the skin (P). On the contrary, the skin vasculature in control embryos is restricted to a two-dimensional network (M,O); n > 5. Visualization of lymphatic vessels (LYVE-1, green) revealed an increase in lymphatic vessels in EXT1MCko embryonic skin (R) compared with control littermates (Q); however, colabeling with endomucin (red) showed separation of lymphatic and blood vessels and no defects in lymphangiogenesis. E indicates epidermis; D, dermis; S, subcutis. n = 2.
) and loose connective tissue in the epidermis of EXT1MCko (F) compared with the control littermate (C,E); n = 2. Endomucin staining (G-N, red) of E13.5 mutant embryos revealed abnormal patterning of the superficial vessels at the back along the spinal cord (G-H). (I-J) Higher-magnification images of the boxed area in panels G and H. The skin vasculature of the control littermates is uniform in its size and diameter (K). In contrast, EXT1MCko display irregular-shaped, tortuous vessels with fluctuation in their diameter (arrowheads, L). EXT1MCko exhibit excessive filopodia extension (N), indicating aberrant sprouting processes, which are reflected in embryonic skin sections showing a widespread network of blood vessels through the entire depth of the skin (P). On the contrary, the skin vasculature in control embryos is restricted to a two-dimensional network (M,O); n > 5. Visualization of lymphatic vessels (LYVE-1, green) revealed an increase in lymphatic vessels in EXT1MCko embryonic skin (R) compared with control littermates (Q); however, colabeling with endomucin (red) showed separation of lymphatic and blood vessels and no defects in lymphangiogenesis. E indicates epidermis; D, dermis; S, subcutis. n = 2.
Detailed whole-mount analysis of these skin areas using endomucin labeling to detect ECs21 reveals striking abnormalities in superficial vessels around the spinal cord in mutant embryos (Figure 3G-N). Whereas control embryos display an organized and hierarchical vascular pattern (Figure 3G), vessels in mutant embryos appear random and chaotically branched with variable diameter (Figure 3H). Higher magnification analysis emphasizes diameter variability (Figure 3J) and shows frequent formation of glomeruloid structures resembling microaneurysms (Figure 3J arrow).
Confocal microscopy confirms the presence of a patent network of vessels in control embryos (Figure 3K), whereas mutant tissue shows slender processes without filopodia resembling regressing vessels (Figure 3L arrowheads), and also numerous new sprouts alongside tortuous vessels of variable caliber (Figure 3N; supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article).
Given that perfusion is a stabilizing factor important for vessel maturation, we studied heart function and major vessel perfusion. Invariably, all mutant and control embryos displayed effective heartbeat and Evans blue or fluorescein isothiocyanate dextran distributed quickly through the major central vessels after injection into the heart (not shown). In addition, observations by light and fluorescence microscopy revealed the presence of erythrocytes in vessels of the capillary plexus in EXT1MCko to the same extent as seen in the vasculature of control embryos, indicating that blood flow is not defective in EXT1MCko. Proper cardiac function beyond E14 requires coronary vessel formation, which is regulated by a series of HS-dependent signaling pathways, including hedgehog, TGF-β, fibroblast growth factor, and vascular endothelial growth factor (VEGF).22 However, coronary vessel formation is unperturbed in EXT1MCko embryos at E13.5 (supplemental Figure 2A-D). Thus, central cardiovascular development and function appear not to require MC HS production. The observed vascular defects in the skin are therefore probably the result of local defects associated with MC HS deficiency.
Confocal three-dimensional analysis illustrates that the vascular network in mutant skin samples spanned a greater depth (Figure 3M-N), possibly because of increased sprouting or because of the tissue swelling and edema. Paraffin cross sections confirm the presence of multiple layers of vascular profiles in the hypodermis (Figure 3O-P).
Edema formation during embryonic development can result from either primary defects in blood vessels, or alternatively, from defects in lymphatic development. Mutations in major signaling pathways relevant for lymphangiogenesis and the separation of blood and lymphatic vessels led to edema formation between E12.5 and 13.5, similar to PC HS mutants. LYVE-1 labeling (a marker for lymphatic endothelium23 ) on sections illustrates an increase in lymphatic vessel profiles in the embryonic skin of EXT1MCko E13.5 embryos compared with control littermates (Figure 3Q-R). Double-labeling with endomucin shows a clear separation of blood and lymphatic vessels. Formation of mayor lymphatic vessels and jugular lymph sacs also appear unaffected in EXT1MCko (supplemental Figure 3A-B). These results indicate that lymphangiogenesis and segregation do not require HS production by MCs and that the observed edema formation is probably caused by increased permeability in the blood vascular compartment.
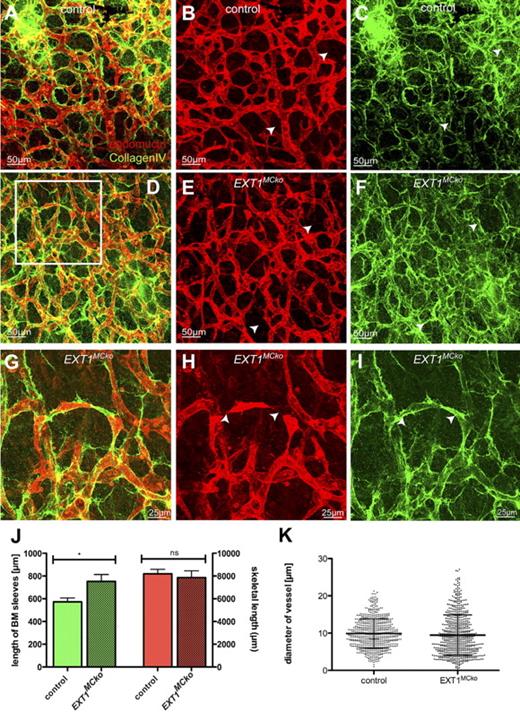
Detailed studies of the blood vessel morphology using endomucin whole-mount labeling reveals a remarkable increase in filopodia extension, indicating increased sprouting activity (Figure 3M-N). This increased sprouting, however, appears not to result in greater vascular density, suggesting that nascent vessels are less stable and prone to regress in the absence of MC HS production. To test this hypothesis, we studied the distribution of the BM component collagen IV (CIV). CIV is a major component of the BM surrounding the mature blood vessel but is absent from the tips of new sprouts. When blood vessels regress, they leave behind empty BM sleeves containing CIV.24 Comparing endomucin and CIV staining thus enables assessment of the relative amount of newly forming and regressing vessel.25
Using this technique, we consistently observe increased CIV staining and numerous CIV-positive structures devoid of endomucin staining, signifying vessel regression. Control embryos also display occasional regression profiles as part of normal vascular remodeling (Figure 4A-C). Quantification of total vessel density by measuring the skeletal length of all endomucin-labeled vessel profiles in confocal z-stacks shows no significant difference between mutants and controls (Figure 4J). Quantification of regression profiles in relation to the total skeletal length of vessels, however, reveals a significant increase in the length and number of CIV sleeves in mutant tissue (Figure 4J), indicating that loss of MC HS leads to increased vessel regression, and compensatory new sprouting, together culminating in an overall vessel density that is comparable with controls. Higher magnification frequently shows fragmented sprouts, apparently failing to form a patent stalk (Figure 4G, boxed area in Figure 4D), as well as a conspicuous variability in vessel diameter. Quantitative assessment of the vessel diameter reveals an identical mean vessel diameter in mutant and control tissues. However, a significant increase in the SD illustrates that the loss of MC HS results in increased diameter variability (Figure 4K). Together, these results suggest that the loss of MC HS compromises stability and diameter control of nascent vessels, promoting both increased regression and sprouting.
MC HS is important for stability and diameter control of vessels in the skin. Endomucin (red) and collagen IV (green) staining of embryonic skin revealed regressing blood vessel in EXT1MCko embryos compared with control littermates (A-I). White arrowheads represent regressing vessels lacking EC marker endomucin but positively stain for BM component CIV. Higher magnification images (G-I) of the boxed area in panel D show disconnected ECs that lack proper formation of lumen (arrowheads). Quantification for CIV-positive and endomucin-negative vessels confirmed significant increase in regressing blood vessels of EXT1MCko (J), whereas the density of the vascular network in the skin is unaffected (J, red bars). (J) n > 15 images/embryo; 2 embryos/group. Measurement of vessel diameter reveals a significant increase in diameter variability, whereas the mean diameter is identical between control and mutant samples. This conspicuous variation in vessel diameter was consistently observed in all mutant samples; n > 5. (K) Measurement of 650 vessel profiles (n > 15 images/embryo), reflecting the variability in vessel diameter in EXT1MCko embryos; n = 2. ***F < 0.0001 (F test, variance of SD). ns indicates not significant.
MC HS is important for stability and diameter control of vessels in the skin. Endomucin (red) and collagen IV (green) staining of embryonic skin revealed regressing blood vessel in EXT1MCko embryos compared with control littermates (A-I). White arrowheads represent regressing vessels lacking EC marker endomucin but positively stain for BM component CIV. Higher magnification images (G-I) of the boxed area in panel D show disconnected ECs that lack proper formation of lumen (arrowheads). Quantification for CIV-positive and endomucin-negative vessels confirmed significant increase in regressing blood vessels of EXT1MCko (J), whereas the density of the vascular network in the skin is unaffected (J, red bars). (J) n > 15 images/embryo; 2 embryos/group. Measurement of vessel diameter reveals a significant increase in diameter variability, whereas the mean diameter is identical between control and mutant samples. This conspicuous variation in vessel diameter was consistently observed in all mutant samples; n > 5. (K) Measurement of 650 vessel profiles (n > 15 images/embryo), reflecting the variability in vessel diameter in EXT1MCko embryos; n = 2. ***F < 0.0001 (F test, variance of SD). ns indicates not significant.
The precise mechanism of blood vessel regression in vivo is not fully understood. It is conceivable that increased sprouting and concomitant regression would correlate with an increase in EC turnover. We therefore performed TUNEL assay and caspase 3 staining to detect cell death, as well as phospho-histone H3 staining to analyze proliferation. Intriguingly, neither cell death nor proliferation is significantly changed in HS mutants during various stages of embryonic development (supplemental Figures 4-5), suggesting that migration/rearrangement of existing cells contributes substantially to the remodeling process.
Loss of MC HS results in reduced MC coverage in the skin
Increased vessel permeability and regression can directly result from PC loss, both during development and in disease processes, such as diabetic retinopathy.9,26-28 To address whether the regression of vessels and vascular dysmorphogenesis is caused by MC detachment/loss, we visualized MCs in skin samples using NG2.20
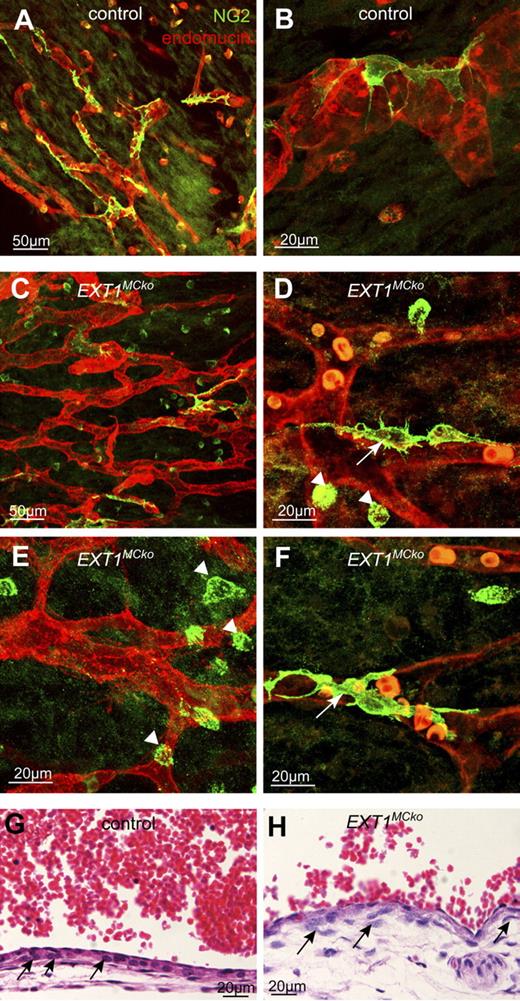
In control embryos MCs are closely attached to the endothelium, covering almost the entire vascular network (Figure 5A-B). In EXT1MCko skin samples from identical regions, however, MC coverage is clearly reduced, with many vessels lacking properly attached MCs (Figure 5C-F). In addition to MCs in contact with the endothelium (Figure 5D,F arrows), NG2 antibodies labeled isolated cells situated in between vessels (arrowheads). In control samples, such cells are few and far between, and most of these appear spindle-shaped. In contrast, isolated NG2-positive cells in mutant tissues are much more abundant and conspicuously round. Such isolated NG2-positive cells could represent MCs progenitor cells not yet recruited to the vessels, or MCs that have detached. Detached MCs in the CNS of PDGF-B retention mutants or Ndst1−/− mice usually appear spindle-shaped or extend multiple processes but do not appear round. TUNEL analysis and caspase 3 detection do not label these cells, indicating that the rounded appearance is not associated with cell death. Whether these cells represent MC progenitor cells that fail to be recruited remains to be determined.
Recruitment and attachment defects of HS-deficient MCs in embryonic skin. MCs of the skin vasculature were visualized with NG2 (green, A-F) in E13.5 control (A-B) and EXT1MCko embryos (C-F). Blood vessels in the mutant display a reduced number of MCs in the vascular plexus (C) compared with the control (A). White arrows represent MCs that attach to the vessel and are in close contact to the endothelium (red; D,F) as seen similarly in the control (B). However, EXT1MCko vessels are dominated by rounded NG2-positive cells in close proximity to the vessel (arrowhead; D-E), indicating progenitor cells that fail to differentiate; n > 5. Embryonic transverse section (G-H) confirms the presence of MCs; however, MC organization is chaotic (H,  ) compared with the tight alignment of MC in control cardinal vein (G).
) compared with the tight alignment of MC in control cardinal vein (G).
Recruitment and attachment defects of HS-deficient MCs in embryonic skin. MCs of the skin vasculature were visualized with NG2 (green, A-F) in E13.5 control (A-B) and EXT1MCko embryos (C-F). Blood vessels in the mutant display a reduced number of MCs in the vascular plexus (C) compared with the control (A). White arrows represent MCs that attach to the vessel and are in close contact to the endothelium (red; D,F) as seen similarly in the control (B). However, EXT1MCko vessels are dominated by rounded NG2-positive cells in close proximity to the vessel (arrowhead; D-E), indicating progenitor cells that fail to differentiate; n > 5. Embryonic transverse section (G-H) confirms the presence of MCs; however, MC organization is chaotic (H,  ) compared with the tight alignment of MC in control cardinal vein (G).
) compared with the tight alignment of MC in control cardinal vein (G).
We further noted that MCs surrounding the cardinal vein at E16.5 also appear disorganized and more rounded in EXT1MCko embryos, losing the neat stratification observed in littermate controls (Figure 5G-H black arrows). Thus, it is possible that MCs that are not in direct cell-cell contact with ECs require cell-autonomous HS production for proper polarization, recruitment, and organization.
Mice lacking MC HS display reduced phosphorylation levels of PDGFR-β and SMAD
To gain a better understanding of potential signaling pathways affected by MC HS depletion, we analyzed protein expression and activation of growth factor receptors as well as downstream signaling molecules of signaling pathways that have been described in previous studies to be dependent on HS-mediated binding and interaction. To this end, we dissected samples of embryonic back skin for protein analysis.
Phosphorylation of PDGFR-β is reduced in EXT1MCko skin samples (Figure 6A), consistent with the observed reduction in MC coverage. Phospho-Erk1/2 and phospho-SHP-2, which relay signaling downstream of PDGFR-β, are consistently reduced, whereas p-Akt signaling is unaffected (Figure 6A). Densitometric analyses summarize the reduction of p-PDGFR-β in the skin but reveal no significant defects in p-PDGFR-β and its downstream targets in protein samples of the CNS. Thus, the biochemical data are consistent with the observed phenotype in the skin and with the lack of an MC phenotype in the CNS.
Growth factor signaling in mice lacking MC HS. Phospho-PDGFR-β protein expression levels as well as downstream signaling molecules p-Erk1/ 2 and p-SHP-2 are reduced in skin samples of several EXT1MCko, whereas p-Akt signaling is unaffected (A). Comparison of pPDGFR-β levels in skin and CNS of EXT1MCko confirmed that PDGFR-β signaling is unchanged in the CNS of EXT1MCko mice (A) correlating with the observed phenotype; illustration of the percentage of pPDGFR-β normalized to ribosomal protein S6, internal loading control in the skin and brain of protein samples. *P < .05. Control and EXT1MCko; n ≥ 3. Values are mean ± SEM. Immunoprecipitation of VEGFR2 followed by immunoblotting to detect phosphorylated VEGFR2 (B) revealed no differences in pVEGFR2 expression levels in the skin of EXT1MCko; n = 2. Expression studies of pSMAD2 and pSMAD3 molecules by Western blotting, downstream effectors of the TGF-β pathway revealed a significant down regulation in EXT1MCko embryos (C). The reduction in pSMADs is restricted to EXT1MCko skin samples, whereas pSMAD levels in the CNS of mice lacking HS in MCs are unchanged compared with control littermates (C). To access whether reduced pSMAD levels are a result of MC loss in the skin of EXT1MCko embryos, we analyzed pSMAD expression in Pdgfrb null mice lacking MC investment (C). (D) Summarization of densitometric analyses, illustrating the expression levels of pSMAD2 and pSMAD3 normalized to β-tubulin, internal loading control. *P < .05. ns indicates not significant. EXT1MCko and control embryos (n = 4), Pdgfrβ+/− and Pdgfrβ−/− (n = 2). Values are mean ± SEM. Reduced endothelial pSMAD levels could be confirmed by immunohistochemical staining with a pSMAD3 antibody on embryonic skin sections (E-H). Whereas most ECs of blood vessels in control mice are stained positively for pSMAD3 (arrows; E-F), only few ECs in EXT1MCko express pSMAD3 expression (arrows, G-H). Counterstaining with endothelial marker endomucin (red) confirmed endothelial-specific loss of pSMAD3 (green) in EXT1MCko embryos (arrows, G-H), reflecting the reduction of pSMAD3 protein levels in skin (D); n > 3; illustration of different modes of MC recruitment in skin and CNS (I).
Growth factor signaling in mice lacking MC HS. Phospho-PDGFR-β protein expression levels as well as downstream signaling molecules p-Erk1/ 2 and p-SHP-2 are reduced in skin samples of several EXT1MCko, whereas p-Akt signaling is unaffected (A). Comparison of pPDGFR-β levels in skin and CNS of EXT1MCko confirmed that PDGFR-β signaling is unchanged in the CNS of EXT1MCko mice (A) correlating with the observed phenotype; illustration of the percentage of pPDGFR-β normalized to ribosomal protein S6, internal loading control in the skin and brain of protein samples. *P < .05. Control and EXT1MCko; n ≥ 3. Values are mean ± SEM. Immunoprecipitation of VEGFR2 followed by immunoblotting to detect phosphorylated VEGFR2 (B) revealed no differences in pVEGFR2 expression levels in the skin of EXT1MCko; n = 2. Expression studies of pSMAD2 and pSMAD3 molecules by Western blotting, downstream effectors of the TGF-β pathway revealed a significant down regulation in EXT1MCko embryos (C). The reduction in pSMADs is restricted to EXT1MCko skin samples, whereas pSMAD levels in the CNS of mice lacking HS in MCs are unchanged compared with control littermates (C). To access whether reduced pSMAD levels are a result of MC loss in the skin of EXT1MCko embryos, we analyzed pSMAD expression in Pdgfrb null mice lacking MC investment (C). (D) Summarization of densitometric analyses, illustrating the expression levels of pSMAD2 and pSMAD3 normalized to β-tubulin, internal loading control. *P < .05. ns indicates not significant. EXT1MCko and control embryos (n = 4), Pdgfrβ+/− and Pdgfrβ−/− (n = 2). Values are mean ± SEM. Reduced endothelial pSMAD levels could be confirmed by immunohistochemical staining with a pSMAD3 antibody on embryonic skin sections (E-H). Whereas most ECs of blood vessels in control mice are stained positively for pSMAD3 (arrows; E-F), only few ECs in EXT1MCko express pSMAD3 expression (arrows, G-H). Counterstaining with endothelial marker endomucin (red) confirmed endothelial-specific loss of pSMAD3 (green) in EXT1MCko embryos (arrows, G-H), reflecting the reduction of pSMAD3 protein levels in skin (D); n > 3; illustration of different modes of MC recruitment in skin and CNS (I).
Jakobsson et al previously showed ex vivo that MC HS is sufficient to induce vessel formation and to support VEGF signaling during sprouting angiogenesis in three-dimensional cultures of embryoid bodies.29 We therefore hypothesized that VEGF signaling might be affected on deletion of pericytic HS in vivo, possibly leading to the observed alteration in sprouting and regression. However, immunoprecipitation of VEGFR-2 using total embryonic tissue followed by detection with a phospho-VEGFR-2 antibody reveals no significant differences in VEGFR2 phosphorylation levels in EXT1MCko compared with control littermates (Figure 6B). Overall, VEGFR2 levels are also comparable (supplemental Figure 6), together illustrating that MC HS is not required for VEGF signaling during vessel development in vivo.
Other HS-dependent signaling pathways involved in vascular development and MC recruitment include the TGF-β pathway, involving TGF-β, TGFIIR, Alk1/Alk5, the auxiliary TGF-β receptor endoglin, and the downstream effectors SMAD1, 2, 3, and 5.30-33
TGF-β is involved in local induction of MC from progenitor cells of the mesenchymal cell lineage as well in differentiation and proliferation of ECs and MCs.4,34 Protein analysis of the embryonic skin vasculature reveals reduced phosphorylation of SMAD2, SMAD3 (Figure 6C), and SMAD1 (data not shown). Densitometric quantification illustrates that, similar to the tissue-specific deficiency in phosphorylation of PDGFR-β, phosphorylated (p)SMAD2 and pSMAD3 levels are significantly reduced in the skin, but not in the brain of EXT1MCko embryos (Figure 6C). Control littermates show comparable pSMAD2 and pSMAD3 levels in skin and brain. To assess whether the reduced pSMAD level is a consequence of reduced MC recruitment rather than HS function, we studied pSMAD levels in skin samples of pdgfrb−/− embryos, which have substantially reduced MC recruitment (Figure 6C). Quantification revealed no significant differences between samples from control (Pdgfrb+/−) and Pdgfrb−/− embryos, although there was a tendency toward reduced pSMAD3 levels in the brain (Figure 6D).
To confirm these findings at the cellular level, we visualized pSMAD3 in the dermal vasculature of embryos. Immunohistochemical and immunofluorescent staining reveals that most endothelial as well as some perivascular cells express pSMAD3 in control embryos (arrows, Figure 6E-F, supplemental Figure 7A-B). In contrast, ECs lining the blood vessels (indicated by asterisk) of EXT1MCko embryos frequently lacked pSMAD3 expression (supplemental Figure 7C-D arrows). Counterstaining with endomucin to identify ECs (Figure 6E-H) revealed cell type-specific loss of endothelial pSMAD3 in capillaries of EXT1MCko skin samples compared with control embryos. Arrows indicate reduced number of pSMAD3-positive ECs (Figure 6G-H arrows), indicating that MC HS plays a role in TGF-β signaling between MCs and the endothelium.
Discussion
The present study underscores the fundamental importance of HS in tissue biology. Selective deletion of all HS in MC is incompatible with embryonic survival because of an important role of MC HS during the late stages of vascular morphogenesis and stability.
ECs and PCs share a BM, and both cell types potentially contribute to the establishment thereof by production of various ECM proteins, including HSPGs. The vascular BM at the interface between ECs and MCs is rich in N-sulfated HS. Reduced N-sulfation impairs PDGF-BB binding and PDGFR-β signaling, leading to delayed PC recruitment and increased detachment.1,12 Complete loss of N-sulfated HS in vascular structures growing from embryoid bodies ex vivo illustrated that VEGFR signaling requires HS as coreceptor during vascular morphogenesis. In the absence of endothelial HS, MC-derived N-sulfated HS is sufficient to support VEGFR signaling, illustrating that HS and VEGFR can interact in trans during this process.29
Our genetic approach to generate EXT1MCko embryos effectively depleted HS from MCs during vascular development. Major vascular defects associated with MC deficiencies occur in EXT1MCko embryos selectively outside of the CNS, most notably along the embryonic back skin at stages after E12.5. Edema and swelling, as well as hemorrhaging, indicate profound vascular dysfunctions as a result of HS deficiency. Histologic sections confirm edema formation despite abundant lymphatic vessel development. Central lymphatic development, including segregation of jugular lymph sacs from the vein, appear unaffected, suggesting that the edema is a result of increased vascular permeability and not of defective lymphangiogenesis.
Whole-mount analysis of EXT1MCko skin samples at E13.5 and later illustrates increased vascular diameter irregularities and frequent regression profiles, as well as excessive sprouting. These abnormalities are similar to those caused by MC deficiencies in Pdgfb or Pdgfrb knockouts. Pericyte/MC detection by NG2 staining confirms a profound reduction in MC coverage in the affected vascular plexus areas. At the same time, conspicuously rounded NG2-positive cells are found in close proximity to many skin vessels. We speculate that the concomitant appearance of these cells and the apparent loss of PCs may signify a defect in recruitment of local progenitor cells. LeJan and Kreuger (Karolinska Institutet, Stockholm, Sweden, personal oral communication, September 2008) observed a strikingly similar rounded PC phenotype in embryoid bodies deficient in EXT1, suggesting that the HS may be cell-autonomously required for local induction and/or polarization and spreading of MC progenitor cells. In line with this idea, we observed defective alignment of HS-deficient MCs around the aorta and cardinal vein in EXT1MCko embryos.
Several studies have highlighted the roles for Syndecan-1, a transmembrane HSPG in cell spreading, actin bundling, and cell migration.35 In addition, epithelial cell polarization in organotypic cultures is mediated by laminin and HSPG interaction. Isolated NG2-positive cells were also found in the skin of Pdgfrb−/− embryos (data not shown), again correlating with a severe loss of MC on vascular profiles. Protein lysates from EXT1MCko and control skin samples revealed a strong reduction in phosphorylated PDGFR-β, and loss of SHP2 and Erk1/2 activation. Similar to the results obtained in Ndst1−/− MEFs,1 Akt activation was not affected. Thus, unlike brain PCs, PCs in the periphery require HS production for effective PDGFR-β signaling and recruitment to the vessel wall. The differential requirement of HS could be related to different modes of recruitment. Longitudinal recruitment of PCs during angiogenic sprouting in the CNS relies on migration and proliferation of PCs along the abluminal endothelial surface.36 This process depends on HS-mediated PDGF-BB retention close to the endothelial surface.1,11 Arguably, endothelial HS production and deposition in the endothelial BM would be ideally suited to control PDGF-BB retention and function as coreceptor for pericytic PDGFR-β activation in trans. In contrast, MC progenitor cells that reside at a distance from the vessel may cell-autonomously require HS on the cell surface for activation in cis. Recruitment of MC to vessels in the skin involves induction of new progenitor cells from mesenchymal lineages.3 Thus, the spatial relationship between signal sending cell (the endothelium) and signal receiving cell (the MCs) is very different during longitudinal recruitment in the CNS and local progenitor induction in the periphery (Figure 6I). In the former case, migration will occur along an HS-rich BM even when MCs themselves do not produce HS. In the latter, however, recruitment will require polarization toward the signal, and migration over variable distances and the attractive signal may require transport or diffusion across several neighboring cells. It is possible that this particular spatial constellation requires cell-autonomous HS production by MCs or their progenitors. An alterative explanation could be that CNS MCs require fundamentally different signals than peripheral MCs, possibly because they derive from distinct lineages. Indeed, lineage-tracing studies indicated that MCs in the brain derive from neural crest, whereas MCs in the periphery are of mesodermal origin.37 However, it seems improbable that PDGFR-β signaling differentially relies on HS in neural crest vs mesenchymal cell populations. Rather, the proximity to normal endothelial BM and the juxtacrine vs paracrine spatial configuration represent a more plausible explanation for the observed differences.
Ex vivo studies using endothelial and 10T1/2 cell cocultures illustrated that, in addition to PDGF-BB/PDGFR-β signaling, TGF-β signaling is involved in PC recruitment and differentiation.4,34,38 Defective vascular development associated with reduced MC recruitment and impaired vessel stability is observed in mouse mutants of TGFRII, Alk1, Alk5, endoglin, and SMAD1/5.39 Similar defects are observed in patients with hereditary telangiectasia, a genetic disease associated with loss-of-function mutations in TGF-β pathway components.40 TGF-β is expressed by both ECs and MCs, and reciprocal signaling regulates MC induction from mesenchymal progenitors and various EC functions, including expression of stabilizing matrix components, cell proliferation, and differentiation. The observed profound reduction in SMAD1, 2, and 3 phosphorylation in Western blots of skin samples, and in ECs identified on skin sections, may indicate that endothelial TGF-β signaling is defective in the absence of MC HS. In contrast, brain samples from the same embryos show no defects in SMAD phosphorylation. Thus, the loss of HS appears to impair both TGF-β and PDGFR-β signaling selectively in the skin, but not in the brain.
In addition to the direct binding of TGF-β dimers to HS, recent work illustrated that HS can play an important role in linking the latent TGF-β–binding protein LTBP1 to fibronectin, providing a mechanism for TGF-β storage in the ECM.31 In Drosophila wing patterning, a process regulated by gradients of the TGF-β family protein Dpp, HS proved essential for gradient formation by facilitating extracellular transport of Dpp across the epithelial cells.30 Thus, HS could be important for the availability, potential gradient formation, and activity of TGF-β during the induction and recruitment of MCs from mesenchymal progenitors. Finally, the recent observation that TGF-β induces actin reorganization mediated by SMAD 2 and 3 by activating Rho-GTPases,41 could potentially explain the rounded appearance of NG2-positive cells residing in the mesenchyme, as well as changes in EC responses.
In conclusion, the present work provides evidence for a differential involvement of cell-autonomous HS production during PC recruitment in the CNS and in the periphery (Figure 6I). Our results also suggest that the involvement of HS in PDGF-BB and TGF-β receptor activation acting in cis or in trans may be context dependent and will arguably be affected by the distance between the signal sending and receiving cell. For PC/MC biology, the current findings advance our understanding of selective cellular interactions during recruitment and of the importance of MC-derived HSPGs for functional vascular patterning.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank David Ish-Horowicz and Johan Kreuger for valuable comments on the manuscript, Lucy Davinson for proofreading, and Sue Watling, Craig Thrussell, and Claire Darnborough for excellent animal husbandry and help with tissue collection and processing.
This work was supported by Cancer Research UK (London, United Kingdom), the EMBO Young Investigator Program (Heidelberg, Germany), and the Lister Institute of Preventive Medicine (Bushey, United Kingdom).
Authorship
Contribution: D.S. designed and performed experiments, analyzed data, and wrote the paper; E.N. performed experiments; M.N. provided material; R.H.A. and Y.Y. provided transgenic mouse lines; and H.G. designed experiments, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Holger Gerhardt, Vascular Biology Laboratory, London Research Institute–Cancer Research UK, 44 Lincoln's Inn Fields, London, WC2A 3PX, United Kingdom; e-mail: Holger.gerhardt@cancer.org.uk.