Abstract
The transcription factor RUNX1/AML1 is a master regulator of hematopoietic development. Its spatiotemporal expression is tightly regulated during embryonic development and is under the control of 2 alternative promoters, distal and proximal. Despite the functional significance of Runx1, the relative and specific activities of these 2 promoters remain largely uncharacterized. To investigate these activities, we introduced 2 reporter genes under the control of the proximal and distal promoters in embryonic stem cell and transgenic mouse lines. Our study reveals that both in vitro and in vivo the proximal Runx1 isoform marks a hemogenic endothelium cell population, whereas the subsequent expression of distal Runx1 defines fully committed definitive hematopoietic progenitors. Interestingly, hematopoietic commitment in distal Runx1 knockout embryos appears normal. Altogether, our data demonstrate that the differential activities of the 2 Runx1 promoters define milestones of hematopoietic development and suggest that the proximal isoform plays a critical role in the generation of hematopoietic cells from hemogenic endothelium. Identification and access to the discrete stages of hematopoietic development defined by the activities of the Runx1 promoters will provide the opportunity to further explore the cellular and molecular mechanisms of hematopoietic development.
Introduction
The first hematopoietic cells found in the mouse embryo, primitive erythrocytes, emerge in the yolk sac blood islands between the primitive streak and 20 somite-pair (sp) stages of development. This transient wave of primitive erythrocyte generation, defined as primitive hematopoiesis, is shortly followed by the production of definitive hematopoietic progenitors in the yolk sac.1 Definitive hematopoiesis encompasses by convention the development of all lineages other than primitive erythroid cells and includes definitive erythroid, myeloid, and lymphoid lineages. On the basis of the close association of hematopoietic cells with endothelial cells in the blood islands, it was suggested that both lineages derive from a common precursor, the hemangioblast.2,3 The hematopoietic differentiation of embryonic stem (ES) cells in vitro provided the first direct proof for the existence of a hemangioblast, with the identification of a clonal precursor able to generate blast colony containing hematopoietic and endothelial progenitor cells.4 The authors of subsequent studies demonstrated the in vivo existence of the hemangioblast in mouse5 and zebrafish.6
By midgestation, hematopoietic cells are found in clusters tightly associated with the ventral aspect of the dorsal aorta, and the cells in these clusters were shown to be of endothelial origin.7,8 This endothelial/hematopoietic relationship is further supported by the observation that definitive hematopoietic progenitors, of both embryo proper and yolk sac origin, were shown to differentiate from VE-cadherin–positive endothelial cells.9-11 On the basis of these findings it was suggested that definitive hematopoietic cells derive from a specific subset of endothelial cells with hematopoietic potential, a hemogenic endothelium. Recent studies,8,12 such as VE-cadherin lineage tracing, single-cell sorting, and single-cell imaging of blood cell generation by endothelial cells, have definitively established the endothelial origin of at least some hematopoietic cells. Furthermore, we recently demonstrated that the hemangioblast generates hematopoietic cells through a hemogenic endothelium intermediate stage.13
The transcription factor RUNX1/AML1 is a master regulator of hematopoietic development, whose expression and function are associated with the establishment and maintenance of hematopoietic cells. In mice, Runx1 deletion results in midgestation lethality14,15 and a complete block in development of definitive hematopoietic progenitors.14,16,17 RUNX1 activity has been shown to be more specifically required for the generation of these precursors in endothelial cells characterized by Tie2 and VE-cadherin expression.10,18,19 Although Runx1 is clearly critical for the establishment of definitive hematopoiesis, knockout of Runx1 in adult mice leads in contrast to a mild phenotype as the animals survive with relatively normal numbers of hematopoietic cells.20,21
In vertebrates, the transcription of the Runx1 gene is under the control of 2 alternative promoters, distal P1 and proximal P2, which generate distal and proximal Runx1 transcripts that differ in their 5′ untranslated regions (UTRs) and N-terminal coding sequences, and that at full length produce the RUNX1c and RUNX1b protein isoforms, respectively.22-24 Specific alternative splicing of the proximal or the distal transcripts generates additional RUNX1 protein isoforms.25-28 In mouse embryonic hematopoiesis, the proximal Runx1 mRNA is dominant until E10.5, whereas by midgestation and in adult mice the majority of Runx1 mRNAs detected in hematopoietic cells initiate from the distal promoter.26,28 During embryogenesis in zebrafish, both promoters were shown to be active but at different sites of definitive hematopoietic development.29 However, a similar transgenic promoter approach in mice failed to indicate that minimal P1 and P2 promoter fragments confer any reproducible hematopoietic specific activity.28 Functionally, it was shown that the selective attenuation of the proximal promoter in mice results in impaired fetal thymus and liver development and neonatal lethality.30 In contrast, the relevance of transcription initiated from the distal Runx1 promoter remains unknown.
Despite these previous findings, the relative and specific activities of the alternative Runx1 promoters during mouse embryonic hematopoiesis remain largely unknown. To investigate the activities of distal and proximal Runx1 promoters at the single-cell level and track the cell populations expressing respective isoforms, we generated mouse ES-cell lines with green fluorescent protein (GFP) and truncated human CD4 (hCD4) reporter genes knocked into the distal and proximal Runx1 promoters, respectively. Our data show that the early expression of the proximal Runx1 isoform is associated with a hemogenic cell population, whereas the subsequent onset of transcription of distal Runx1 coincides with the loss of endothelial phenotype and the appearance of definitive hematopoietic progenitors. The distal-GFP–positive cell population is highly enriched in definitive progenitors during ES-cell differentiation in vitro, as well as in the mouse embryos. Surprisingly, hematopoietic commitment in embryos lacking the distal isoform of Runx1 appeared normal. Altogether, our data demonstrate that the differential activities of Runx1 promoters mark distinct stages of hematopoietic specification.
Methods
Generation of distal-GFP knock-in and distal-GFP proximal-hCD4 double knock-in ES-cell and mouse lines
The homology arms for the targeting vectors were amplified with Phusion DNA Polymerase (NEB/Finnzymes). Detailed description of cloning steps and sequences of primers used to amplify the homology arms are available upon request. Ainv18 mouse ES cells31 were electroporated with linearized targeting vectors and selected with G418. Correctly targeted clones were identified by polymerase chain reaction (PCR), confirmed by Southern blot analysis, and the LoxP-NeoR-TK-PGK-LoxP cassette was removed by transiently expressed Cre recombinase. To generate corresponding mouse lines, correct distal-GFP and double knock-in (KI) clones were injected into blastocysts. The animals in subsequent generations were crossed with C57BL/6 mice. All animal work was performed under regulation governed by the Home Office Legislation under the Animal Scientific Procedures Act (ASPA) 1986 and was approved by the British Home Office.
ES-cell culture and embryoid body differentiation
Ainv18 and Ainv18-derived mouse ES-cell lines were maintained and differentiated as described.32 For reaggregation, sorted cells were cultured in ultra low attachment plates (Costar) in the embryoid body (EB) differentiation media.
Blast colony and hemogenic endothelium cultures
To study blast colony development, fetal liver kinase-1(Flk-1)+ cells were sorted from day 2.5 to 3.0 EBs and plated in gelatin-coated plates or flasks in the BL-CFC media as described previously.13 The hemogenic endothelium cultures were started from different subpopulations of c-Kit+ cells sorted from day 2 blast colony cultures. The cells were grown in gelatin-coated plates as described previously.13
Embryo generation
Timed matings were set up between distalGFP/+ proximalhCD4/+ or distalGFP/GFP male mice and wild-type ICR female mice or between distalGFP/+ mice. Gastrulating embryos were staged by morphologic landmarks.33
Coculture of embryonic cells with OP9 and OP9-DL1 cells
Cells sorted from distalGFP/+ embryos were plated on mouse OP9 or OP9-DL1 stromal cells in α-minimum essential medium supplemented with 20% fetal bovine serum, interleukin-7 (5 ng/mL), Flt3L (5 ng/mL), penicillin, and streptomycin. Cells were harvested every fourth day and stained for lymphoid surface markers and/or replated onto fresh OP9 or OP9-DL1 cells for further culture.
Colony-forming assay
For hematopoietic progenitor assay, cells were plated in 1% methylcellulose as previously described.32 Colonies were scored according to their morphology. Definitive colonies represent all definitive types of hematopoietic colonies, including macrophage, erythrocyte–macrophage, erythrocyte–megakaryocyte, granulocyte–macrophage, and mixed colonies. Data are shown as the mean number of colonies from 3 dishes, and bars represent the standard deviation of the mean.
Flow cytometry and cell sorting
Single-cell suspensions from EBs, embryos, fetal organs, hematopoietic colonies, or OP9 cocultured cells were stained and analyzed with FACSCalibur (BD Biosciences) or sorted with FACSAria or FACSVantage (BD Biosciences). Monoclonal antibodies and streptavidin were from Caltag: human CD4-PE or -bio; from BD Pharmingen: CD4-PE, CD31-bio (MEC13.3), Ter119-PE; or from eBioscience: Flk-1-PE or -APC (Avas12a1), CD41-PE, -bio or -FITC (MWReg30), Mac1-APC (M1/70), CD19-PE (MB19-1,), CD8a-APC (53-6.7), CD45-bio (30-F11), CD34-APC (RAM34), c-Kit-APC (2B8), Tie2-PE (TEK4), AA4.1-APC, Sca1-PE (D7), and streptavidin-PECy7.
Gene expression analysis
Total RNA was extracted with the RNeasy Mini kit and reverse transcribed with random hexamer by the use of the Omniscript RT kit (QIAGEN). The semiquantitative PCRs were performed by the use of GoTaq Mix (Promega) and 0.2μM of each primer. Real-time PCR was performed on ABI 7900 system (Applied Biosystems) with the Exiqon universal probe library. The sequences of all primers are available upon request.
Results
Generation of a distal-GFP proximal-hCD4 double KI ES-cell line
The 2 Runx1 promoter regions generate specific distal and proximal transcripts, which encode, along others, the protein isoforms RUNX1c and RUNX1b, respectively. These 2 protein isoforms differ only by the first 19 (5 different and 14 additional) amino acids at the N terminus of RUNX1c (Figure 1A). We analyzed the presence of distal and proximal Runx1 transcripts during in vitro ES-cell differentiation toward hematopoiesis. Proximal Runx1 transcripts were detected from day 0 to 7 of EB differentiation (Figure 1B). A significant up-regulation of mRNA level was observed between days 2 and 4.5 and was followed by a slow down-regulation at later stages. In contrast, distal Runx1 transcripts were only detected from day 4 of differentiation with increasing level at later stages.
Expression of proximal and distal Runx1 during ES/EB differentiation. (A) Schematic representation of differences in the N-terminal amino acid sequences between distal and proximal Runx1 isoforms. The N-terminally located Runt domain binds DNA and core binding factor-β subunit. (B) Semiquantitative reverse-transcription PCR analysis of expression of distal and proximal Runx1 during ES/EB differentiation. (C) Schematic representation of Runx1 WT allele and double KI allele. Gray and white boxes indicate positions of Runx1 coding and noncoding parts, respectively. Arrows mark positions of PCR primers used for specific detection of both isoforms. (D) Fluorescence-activated cell-sorting (FACS) analysis of Flk-1 and proximal-hCD4 expression during EB differentiation between days 2 and 3.5. (E) FACS analysis of CD41 and proximal-hCD4 expression during EB differentiation between days 3 and 6. (F) FACS analysis of CD41 and distal-GFP expression during EB differentiation between days 4 and 6. (G left) FACS analysis of proximal-hCD4 and distal-GFP expression in presort (P1) and sorted populations (P2-P4) from day 6 EBs. (Middle) Numbers of hematopoietic colonies generated in methylcellulose by populations P1 to P4 from day 6 EBs. Error bars indicate SD of the mean (n = 3). (Right) FACS analysis of CD45 and distal-GFP expression 7 days after replating in methylcellulose media. Numbers represent percentages of respective populations.
Expression of proximal and distal Runx1 during ES/EB differentiation. (A) Schematic representation of differences in the N-terminal amino acid sequences between distal and proximal Runx1 isoforms. The N-terminally located Runt domain binds DNA and core binding factor-β subunit. (B) Semiquantitative reverse-transcription PCR analysis of expression of distal and proximal Runx1 during ES/EB differentiation. (C) Schematic representation of Runx1 WT allele and double KI allele. Gray and white boxes indicate positions of Runx1 coding and noncoding parts, respectively. Arrows mark positions of PCR primers used for specific detection of both isoforms. (D) Fluorescence-activated cell-sorting (FACS) analysis of Flk-1 and proximal-hCD4 expression during EB differentiation between days 2 and 3.5. (E) FACS analysis of CD41 and proximal-hCD4 expression during EB differentiation between days 3 and 6. (F) FACS analysis of CD41 and distal-GFP expression during EB differentiation between days 4 and 6. (G left) FACS analysis of proximal-hCD4 and distal-GFP expression in presort (P1) and sorted populations (P2-P4) from day 6 EBs. (Middle) Numbers of hematopoietic colonies generated in methylcellulose by populations P1 to P4 from day 6 EBs. Error bars indicate SD of the mean (n = 3). (Right) FACS analysis of CD45 and distal-GFP expression 7 days after replating in methylcellulose media. Numbers represent percentages of respective populations.
To follow distal and proximal Runx1 transcription at the single-cell level, we established ES-cell lines containing reporter genes for both distal and proximal Runx1 expression. The GFP gene was first inserted by homologous recombination under the control of Runx1 distal promoter, downstream of the distal 5′ UTR at the level of the first coding ATG of Runx1c (Figure 1C; supplemental Figure 1A, available on the Blood website; see the Supplemental Materials link at the top of the online article). The neomycin selection cassette was then excised from correctly targeted clones by Cre-mediated recombination. Subsequently, a truncated human CD4 cDNA (hCD4) was introduced by homologous recombination downstream of the proximal 5′ UTR at the level of the first coding ATG of Runx1b (Figure 1C; supplemental Figure 1B). The neomycin selection cassette was then excised by Cre-mediated deletion in clones selected for the introduction of hCD4 on the same allele as the distal-GFP targeting. The correct genetic modifications in the distal-GFP/proximal-hCD4 double KI ES-cell line were confirmed by southern blots (supplemental Figure 1C-G).
To confirm that the transcription of the KI reporter genes correlates with those of the endogenous Runx1 isoforms, we monitored the levels of GFP and hCD4 mRNA during the course of a 7-day differentiation (supplemental Figure 2A) and compared these expression patterns to those observed for distal Runx1 and proximal Runx1 mRNA (Figure 1B). The transcription of hCD4 followed closely the transcription of proximal Runx1, increasing from day 2 to 3.5 and then gradually decreasing. Likewise, GFP transcripts were detected at the same time as the distal Runx1 transcription around day 4 of EB differentiation, and the levels of both transcripts increased similarly subsequently (supplemental Figure 2A). We next analyzed the expression of both proximal-hCD4 and distal-GFP at the protein level by flow cytometry during the course of EB differentiation (supplemental Figure 2B). The hCD4 protein was first detected by day 2 of EB differentiation, and the frequency of hCD4+ cells peaked around day 4 and decreased at later days. The first emerging distal-GFP+ cells were detected by day 4 of differentiation, and their frequency increased subsequently. Finally, to validate that detection of hCD4 and GFP proteins reflects, respectively, proximal and distal Runx1 transcription, we sorted GFP+, hCD4+GFP−, and hCD4−GFP− cell populations from day 5 EBs and analyzed for proximal and distal Runx1 transcription. As shown in supplemental Figure 2C, distal Runx1 transcription was principally detected in GFP+ cells. Proximal Runx1 transcription was mainly detected in both populations expressing hCD4 (GFP+ and hCD4+GFP−). Altogether, these data demonstrate that in the double-KI ES-cell line the detection of hCD4 and GFP proteins accurately reflects the transcriptional activities from the proximal and distal Runx1 promoters, respectively.
CD41+ cells emerge from proximal-positive cells and give rise to distal-positive cells
Using this newly generated tool, we investigated the relative transcriptional activities of both Runx1 promoters in the context of other markers of hematopoietic development. After ES-cell differentiation, the first proximal-hCD4+ cells appeared at day 2 of EB differentiation within a population of cells stained positively for Flk-1, the receptor 2 for vascular endothelial growth factor (Figure 1D), whose expression marks a subset of mesoderm committed to cardiac, endothelial, and hematopoietic lineages.4,34 By day 3, a population of proximal-hCD4+ cells negative for Flk-1 appeared, and their frequency increased over time. The expression of CD41 (αIIb integrin), marking hematopoietic commitment both in vivo and in vitro,35,36 was first observed at day 3 of EB differentiation, and at this stage of differentiation all CD41+ cells coexpressed proximal-hCD4 (Figure 1E). Subsequently, a fraction of CD41+ cells was found to be proximal-hCD4−. The first clear distal-GFP+ population was observed by days 4 to 5 of EB differentiation; these cells coexpressed CD41, and their frequency progressively increased (Figure 1F). Altogether, these data show that transcription from the proximal Runx1 promoter is first readily detected in a subset of Flk-1+ cells and that the emergence of CD41+ cells is associated with proximal Runx1 promoter activity. The initial detection of distal Runx1 transcription is subsequently correlated with CD41 expression.
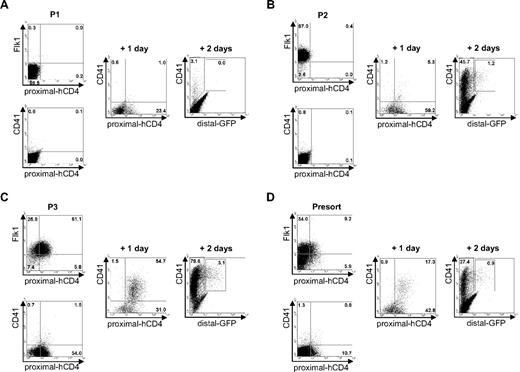
To establish the relationship between the populations defined by the sequential expression of these various markers, we sorted the subpopulations of cells defined by Flk-1 and proximal-hCD4 from day 3 EBs and examined the emergence of CD41 and distal-GFP+ cells upon further culture (Figure 2). After 1 day of culture, Flk-1−/proximal-hCD4− cells (population P1, Figure 2A) gave rise to a subpopulation expressing proximal-hCD4, and the following day CD41+ cells were detected in the culture. The sorted Flk-1+/proximal-hCD4− cells (population P2), negative for CD41 and distal-GFP, generated a high proportion of proximal-hCD4+ cells, with a few coexpressing CD41 after 1 day of culture (Figure 2B and data not shown). One day later, the frequency of CD41+ cells dramatically increased, and distal-GFP+ cells were detected. The third population, Flk-1+/proximal-hCD4+, contained initially only a few CD41+ cells but after 1 day of culture more than 50% of the cells expressed CD41 (Figure 2C). The frequency of these cells further increased during the next 24 hours, at which stage distal-GFP+ cells were also detected. A similar pattern of emergence of the different markers was observed with the nonsorted day 3 EB cells (Presort, Figure 2D). Altogether, these data demonstrate that proximal-hCD4+ cells emerge within a subset of Flk-1+ cells, and that upon further differentiation these Flk-1+/proximal-hCD4+ cells generate CD41+ cells. Later on, part of these newly generated CD41+ cells switch on the expression of distal Runx1.
Relationship between Flk1+, CD41+, proximal-hCD4+, and distal-GFP+ cells. (A-C) FACS profiles of Flk-1, proximal-hCD4, CD41, and distal-GFP expression in populations P1 to P3 sorted from day 3 EBs, analyzed at the day of sort (left), after 1 day of reaggregation (middle), and after 2 days of reaggregation (right). (D) Not-sorted day 3 EB cells FACS analyzed for expression of Flk-1, proximal-hCD4, CD41, and distal-GFP at day 0 (presort) and after reaggregation for 1 or 2 days. Numbers indicate percentages of respective populations.
Relationship between Flk1+, CD41+, proximal-hCD4+, and distal-GFP+ cells. (A-C) FACS profiles of Flk-1, proximal-hCD4, CD41, and distal-GFP expression in populations P1 to P3 sorted from day 3 EBs, analyzed at the day of sort (left), after 1 day of reaggregation (middle), and after 2 days of reaggregation (right). (D) Not-sorted day 3 EB cells FACS analyzed for expression of Flk-1, proximal-hCD4, CD41, and distal-GFP at day 0 (presort) and after reaggregation for 1 or 2 days. Numbers indicate percentages of respective populations.
Distal-GFP+ cells have high definitive hematopoietic potential in vitro
To assess the hematopoietic potential of cells defined by the transcriptional activities of the Runx1 promoters in vitro, we sorted different subpopulations from day 6 EBs on the basis of proximal-hCD4 and distal-GFP detection (Figure 1G left). As expected, the Runx1-negative cells (P2) had very low hematopoietic potential. Primitive erythroid progenitors were detected in both single-positive proximal-hCD4+ (P3) and double-positive proximal-hCD4+/distal-GFP+ (P4) cell populations. In contrast, the double-positive population P4 was highly enriched in definitive hematopoietic precursors (Figure 1G middle). The proximal-hCD4+/distalGFP− cells also gave rise to definitive hematopoietic colonies but with a lower frequency than the P4 population. Furthermore, when analyzed 7 days after replating, all CD45+ hematopoietic cells expressed distal-GFP (Figure 1G right). Together, these observations indicate that the distal-GFP+ cell population is highly enriched in definitive hematopoietic progenitors and that all CD45+ hematopoietic cells have gained expression of distal-GFP during their commitment or maturation.
Expression of proximal-hCD4 specifically marks hemogenic endothelium during blast colony development and in gastrulating embryos
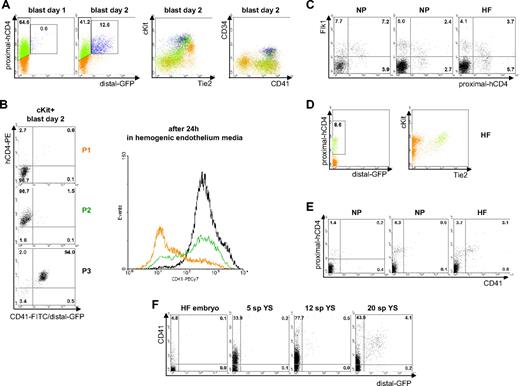
The results described previously indicate that the activity of the proximal Runx1 promoter defines Flk-1+ cells, which subsequently acquire CD41 expression. In contrast, the distal Runx1 promoter appears to be active only afterward, in committed definitive hematopoietic progenitors. We have recently shown that a Tie2+c-Kit+CD41− hemogenic endothelium intermediate population links the generation of CD41+ hematopoietic progenitors to the Flk-1+ hemangioblast cells.13 To gain further insight into the role of Runx1 in that process, we analyzed the expression of proximal and distal Runx1 during these specific stages of blast colony formation. At day 1 of blast colony development, more than half of the cells expressed proximal-hCD4, whereas by day 2 both proximal-hCD4+/distal-GFP− and proximal-hCD4+/distal-GFP+-cell populations were observed (Figure 3A). At this stage, the c-Kit+Tie2+ population was composed of 3 subfractions: double-positive proximal-hCD4+/distal-GFP+, single-positive proximal-hCD4+/distal-GFP−, and double-negative proximal-hCD4−/distal-GFP− (Figure 3A third panel). All double-positive cells were also CD41+ CD34+ (Figure 3A right), indicative of their definitive hematopoietic progenitor nature.37 To determine the hemogenic potential of the 2 other c-Kit+Tie2+ cell populations, we sorted the c-Kit+ fraction of day 2 blast colonies on the basis of hCD4 and CD41/distal-GFP expression (Figure 3B) and cultured the cells in hemogenic endothelium conditions for 24 hours. The proximal-hCD4+/CD41+/distal-GFP+ population (P3), containing committed hematopoietic precursors, was used as a positive control. The proximal-hCD4+/CD41−/distal-GFP− cells (P2) gave rise to CD41+ cells, whereas the majority of proximal-hCD4−/CD41−/distal-GFP− cells (P1) remained CD41− (Figure 3B). Altogether, these data demonstrate that the Tie2+c-Kit+CD41− population contains both proximal-hCD4+ and proximal-hCD4− cells but that the hemogenic potential is specifically enriched in cells where the proximal Runx1 promoter is active.
Proximal-hCD4 expression marks hemogenic endothelium. (A) FACS analyses of proximal-hCD4, distal-GFP, c-Kit, Tie2, CD34, and CD41 expression in blast colonies day 1 and day 2. (B; Left) FACS analysis of proximal-hCD4 and CD41/distal-GFP expression in populations P1 to P3 sorted from c-Kit+ fraction of day 2 blast culture. (Right) CD41 expression in populations P1 to P3 cultured for 24 hours after sorting in hemogenic endothelium media. (C) FACS analysis of Flk-1 and proximal-hCD4 expression in single neural plate (NP) and head fold (HF) embryos. (D) FACS analysis of proximal-hCD4, distal-GFP, c-Kit, and Tie2 expression in representative HF embryo. (E) FACS analysis of proximal-hCD4 and CD41 expression in single embryos. (F) FACS analysis of CD41 and distal-GFP expression in representative single HF embryo and in yolk sacs from embryos between 5 and 20 sp stages. Numbers indicate percentages of respective populations.
Proximal-hCD4 expression marks hemogenic endothelium. (A) FACS analyses of proximal-hCD4, distal-GFP, c-Kit, Tie2, CD34, and CD41 expression in blast colonies day 1 and day 2. (B; Left) FACS analysis of proximal-hCD4 and CD41/distal-GFP expression in populations P1 to P3 sorted from c-Kit+ fraction of day 2 blast culture. (Right) CD41 expression in populations P1 to P3 cultured for 24 hours after sorting in hemogenic endothelium media. (C) FACS analysis of Flk-1 and proximal-hCD4 expression in single neural plate (NP) and head fold (HF) embryos. (D) FACS analysis of proximal-hCD4, distal-GFP, c-Kit, and Tie2 expression in representative HF embryo. (E) FACS analysis of proximal-hCD4 and CD41 expression in single embryos. (F) FACS analysis of CD41 and distal-GFP expression in representative single HF embryo and in yolk sacs from embryos between 5 and 20 sp stages. Numbers indicate percentages of respective populations.
We then asked whether a similar emerging pattern of the different markers and a comparable Tie2+c-Kit+proximal-hCD4+CD41− population could be detected in gastrulating embryos. In individual embryos bearing the double KI allele, proximal-hCD4+ cells were readily detected by the neural plate stage and were mainly associated with Flk-1+ cells (Figure 3C). At the headfold stage, the frequency of proximal-hCD4+ cells negative for Flk-1 increased (Figure 3C), whereas distal-GFP expressing cells could not yet be detected (Figure 3D and data not shown). At this stage, it has been shown that Runx1-positive cells are mostly restricted to yolk sac blood islands and chorionic mesoderm.38 A c-Kit+Tie2+-cell population was observed in which most of the cells were proximal-hCD4+ (Figure 3D) and half of them coexpressed CD41 (Figure 3E), indicating the presence of both Tie2+c-Kit+proximal-hCD4+CD41− and Tie2+c-Kit+proximal-hCD4+CD41+ subpopulations in these headfold embryos. Similar to the data obtained in developing EBs and blast colonies, these first CD41+ cells appeared mainly in the proximal-hCD4+ population, and the first GFP+ cells were observed in vivo within the CD41+ population (Figure 3F). At 10- to 12-sp stage, a small CD41+distal-GFP+ population was detected in the yolk sac (Figure 3F; supplemental Figure 3), and by 20-sp stage such a population was also observed in the embryo proper (supplemental Figure 3). Taken together, these data reveal the dynamic activity patterns of proximal and distal promoters and demonstrate their similarities during in vitro differentiation of ES cells and in gastrulating embryos. Furthermore, these findings indicate that a Tie2+c-Kit+proximal-hCD4+distal-GFP−CD41− population, phenotypically identical to the blast colony hemogenic endothelium, can be identified in headfold-stage individual embryos.
Distal-GFP+ cells have high definitive hematopoietic potential in vivo
To define the biologic potential of cells expressing the distal Runx1 isoform in embryos, we sorted GFP+ and GFP− cells from pooled E8.5 embryos and replated them in conditions supporting the growth of both primitive and definitive colonies. At this developmental stage no separations between yolk sac and embryo proper were performed. As shown in Figure 4A, GFP+ cells were highly enriched in definitive hematopoietic precursors in comparison with GFP− cells. In contrast, the frequency of primitive erythroid precursors was greater in distal-GFP− cells than in distal-GFP+ cells. Interestingly, the morphologies of primitive colonies generated from each population were strikingly different (Figure 4B). Distal-GFP+ cells generated mainly large primitive erythroid colonies, whereas distal-GFP− cells produced principally small colonies. These data indicate that in vivo distal Runx1 is expressed in some early primitive erythroid precursors, but the maturation of these precursors toward erythropoiesis seems to be associated with a rapid loss of its expression. In contrast, the transcriptional activity of the distal promoter of Runx1 is mostly associated with definitive hematopoietic precursors. It should be noted that some reports indicate that the transient primitive wave of hematopoiesis also encompasses some macrophages and megakaryocytes,1,39 suggesting that the definitive colony counts could include precursors of primitive origin which are impossible to distinguish in absence of specific markers. Despite this caveat, it remains clear that all definitive precursors are restricted to the GFP+ cell fraction.
Definitive hematopoietic potential is restricted to distal-GFP+ population. (A) Number of definitive (top) and primitive erythroid (EryP, bottom) colonies generated in methylcellulose by GFP+ and GFP− cells FACS sorted from E8.5 distal-GFP HET embryos. Error bars indicate SD of the mean (n = 3). (B) Pictures of EryP colonies generated from GFP+ and GFP− cells sorted from E8.5 embryos. (C) Number of colonies generated by distal-GFP+, distal-GFP−, and not sorted (pre) cells from E9.5 (top) and E10.5 (bottom) yolk sac (YS) and embryo proper (embryo). (D) Pictures of OP9 cocultures of distal-GFP+ and distal-GFP− cells sorted from E10.5 yolk sac (YS) and embryo proper (embryo), taken 4 days after the sort. (E) FACS analysis of Mac1 and CD19 expression in OP9 cocultures from distal-GFP+ cells taken 8 days after the sort. (F) FACS analysis of CD4 and CD8 expression in OP9-DL1 cocultures from distal-GFP+ cells, taken 13 days after the sort. Numbers indicate percentages of respective populations.
Definitive hematopoietic potential is restricted to distal-GFP+ population. (A) Number of definitive (top) and primitive erythroid (EryP, bottom) colonies generated in methylcellulose by GFP+ and GFP− cells FACS sorted from E8.5 distal-GFP HET embryos. Error bars indicate SD of the mean (n = 3). (B) Pictures of EryP colonies generated from GFP+ and GFP− cells sorted from E8.5 embryos. (C) Number of colonies generated by distal-GFP+, distal-GFP−, and not sorted (pre) cells from E9.5 (top) and E10.5 (bottom) yolk sac (YS) and embryo proper (embryo). (D) Pictures of OP9 cocultures of distal-GFP+ and distal-GFP− cells sorted from E10.5 yolk sac (YS) and embryo proper (embryo), taken 4 days after the sort. (E) FACS analysis of Mac1 and CD19 expression in OP9 cocultures from distal-GFP+ cells taken 8 days after the sort. (F) FACS analysis of CD4 and CD8 expression in OP9-DL1 cocultures from distal-GFP+ cells, taken 13 days after the sort. Numbers indicate percentages of respective populations.
In the next series of experiments, we investigated whether similar results could be observed in E9.5 and E10.5 embryos. At these stages, the transient wave of primitive erythroid precursors is exhausted and the yolk sac–derived progenitors have colonized the embryo proper.1 We observed that GFP+ populations were highly enriched in definitive hematopoietic precursors both in yolk sac and embryo (Figure 4C). Finally, we assayed whether distal-GFP expression was also associated with the acquisition of lymphoid potential. Sorted E10.5 distal-GFP+ and GFP− cells were plated on OP9 and OP9-Delta-like 1 (OP9-DL1) stromal cells in conditions supporting, respectively, the generation of B and T cells. After 4 days, we observed a clear proliferation of the distal-GFP+ cells in both conditions, whereas the distal-GFP− cultures were much more sparse (Figure 4D and data not shown). By day 8, a distinct CD19+Mac1− B-cell population was observed in OP9 cultures seeded with distal-GFP+ cells from both yolk sac and embryo (Figure 4E). Similarly, by day 13 double-positive CD4+CD8+ and single-positive CD4+ or CD8+ T cells were detected in OP9-DL1 cultures seeded with distal-GFP+ cells from both yolk sac and embryo (Figure 4F). Together, these results demonstrate that the transcriptional activity of the distal promoter of Runx1 marks a cell population highly enriched for myeloid, definitive erythroid, and lymphoid potential in E8.5 to E10.5 embryos.
The activities of Runx1 promoters switch during fetal liver hematopoiesis
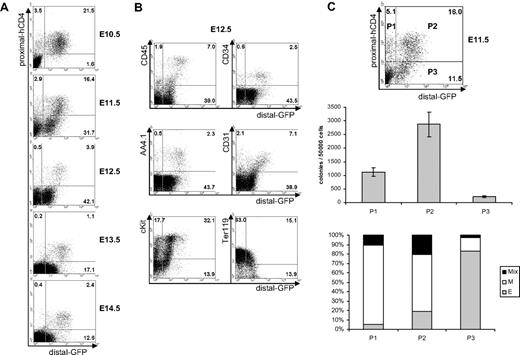
After E10.5 the fetal liver becomes the major site of hematopoiesis within the embryo. To investigate the activity of Runx1 promoters at the fetal liver stage of hematopoietic development, we examined proximal-hCD4 and distal-GFP expression in fetal livers isolated from single embryos between E10.5 and E14.5 (Figure 5A). At E10.5 all Runx1 expressing cells were proximal-hCD4+ and most of them coexpressed distal-GFP. At E11.5 a population of distal-GFP+proximal-hCD4− cells appeared, and by E13.5 to E14.5 all Runx1-positive cells in the fetal liver expressed distal-GFP. From E12.5 onward, most Runx1-expressing cells were distal-GFPlow proximal-hCD4− with only a small fraction distal-GFPhigh and coexpressing proximal-hCD4. To further define the populations expressing different levels of distal-GFP, we stained E12.5 fetal liver cells for CD34, CD45, AA4.1, CD31, and c-Kit, hematopoietic markers known to be expressed by fetal hematopoietic stem and progenitor cells.40-42 Strikingly, most of the distal-GFPhigh cells were CD45+, CD34+, AA4.1+, CD31+, and c-Kithigh. In contrast, the distal-GFPlow cells were mainly negative for these markers but expressed Ter119 (Figure 5B). These results suggest that the maturating Ter119+ erythroid cells quickly lose the expression of Runx1, whereas other hematopoietic cells in the fetal liver maintain Runx1 expression from both promoters.
Expression of proximal-hCD4 and distal-GFP in fetal liver. (A) FACS analysis of proximal-hCD4 and distal-GFP expression in fetal livers isolated from embryos between E10.5 and E14.5. (B) FACS analysis of distal-GFP coexpression with CD45, CD34, AA4.1, CD31, c-Kit, and Ter119 in E12.5 fetal liver. (C top) FACS analysis of prox-hCD4 and distal-GFP expression in E11.5 fetal livers sorted into populations P1 to P3. (C middle) Number of colonies generated in methylcellulose by the FACS sorted populations P1 to P3. Error bars indicate standard deviation of the mean (n = 3). (C bottom) Contribution of erythroid (E), myeloid (M), and mixed (Mix) colonies to the total number of colonies scored for populations P1 to P3 in the colony-forming assay.
Expression of proximal-hCD4 and distal-GFP in fetal liver. (A) FACS analysis of proximal-hCD4 and distal-GFP expression in fetal livers isolated from embryos between E10.5 and E14.5. (B) FACS analysis of distal-GFP coexpression with CD45, CD34, AA4.1, CD31, c-Kit, and Ter119 in E12.5 fetal liver. (C top) FACS analysis of prox-hCD4 and distal-GFP expression in E11.5 fetal livers sorted into populations P1 to P3. (C middle) Number of colonies generated in methylcellulose by the FACS sorted populations P1 to P3. Error bars indicate standard deviation of the mean (n = 3). (C bottom) Contribution of erythroid (E), myeloid (M), and mixed (Mix) colonies to the total number of colonies scored for populations P1 to P3 in the colony-forming assay.
To investigate the hematopoietic potential of the different fetal liver populations defined by the activity of Runx1 promoters, we sorted proximal-hCD4+/distal-GFP− (Figure 5C top), proximal-hCD4+/distal-GFP+, and proximal-hCD4−/distal-GFP+ cell populations from E11.5 fetal liver. The proximal-hCD4+/distal-GFP+ population was enriched in hematopoietic precursors (middle panel). Hematopoietic progenitors were less frequent in the proximal-hCD4+/distal-GFP− population, and these cells, originally distal-GFP−, gave rise to distal-GFP+ cells upon in vitro culture (data not shown). Finally, the proximal-hCD4−/distal-GFP+ cells gave rise to very few colonies, the majority of them being small erythroid colonies (middle and bottom panels). Together, these data indicate that the transcriptional activities of the Runx1 promoters define distinct populations of fetal liver hematopoietic cells: first, a transient proximal-hCD4+/distal-GFP− population; second, a proximal-hCD4+/distal-GFPhigh population, observed at all the time points examined and highly enriched in hematopoietic progenitor cells; and finally, a proximal-hCD4−/distal-GFPlow cell population containing maturating erythroid cells.
Impact of distal Runx1 deletion on hematopoietic development
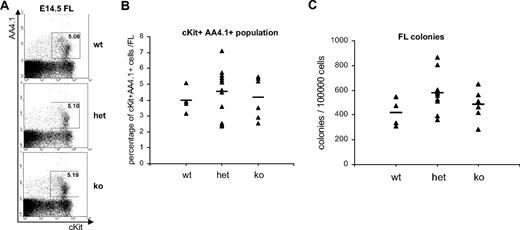
Our data demonstrate that the transcriptional activity of the distal Runx1 promoter marks definitive hematopoietic progenitors from the time of their appearance in the yolk sac at early sp stage to the fetal liver hematopoiesis. On the basis of these data, it seemed likely that the distal isoform of Runx1 plays an important role in the establishment of definitive hematopoiesis. Previous knockouts of Runx1, targeting the Runt DNA binding domain shared by proximal and distal isoforms, did not allow us to distinguish between the specific requirement for transcripts from one or the other promoter. To investigate the outcome of distal Runx1 deletion on the hematopoietic development in mouse embryo, we established a distal-GFP mouse line from the ES-cell line targeted with distal-GFP vector (supplemental Figure 1A), deleting the distal specific coding sequence. After heterozygote matings, wild-type (WT), heterozygote (HET), and knockout (KO) mice were surprisingly born at Mendelian ratios and did not present any specific lethality during adulthood (not shown). These results indicate that the midgestation lethality, characteristic of total Runx1 KO, does not occur in the distal Runx1 KO embryos. When the progenies of heterozygote matings were further analyzed during fetal development, distal Runx1−/− embryos were indistinguishable from WT embryos by gross appearance at all stages examined (not shown), and no defect was observed in fetal liver hematopoiesis. At E14.5, the population of AA4.1+c-Kit+ hematopoietic progenitor cells in the fetal liver did not differ in frequency between WT, HET, and KO embryos (Figure 6A-B). In addition, the average number of hematopoietic colonies generated from distal Runx1+/− and distal Runx1−/− fetal livers did not differ significantly from WT embryos (Figure 6C). Similar analysis of the definitive hematopoietic potential of earlier distal Runx1+/− and distal Runx1−/− embryos (E8.5-E10.5) also failed to reveal any defect in the distal-deficient mice (data not shown). Overall, the hematopoietic development appeared normal in distal-Runx1 KO embryos. Together, these results demonstrate that distal RUNX1 is largely dispensable for the development of the hematopoietic system and for its maintenance in adult animals.
Fetal liver hematopoiesis in distal Runx1-deficient embryos. (A) FACS analysis of AA4.1 and c-Kit expression in E14.5 fetal livers (FL) isolated from distal+/+ (wt), distal+/− (het), and distal−/− (ko) embryos. (B) Percentage of c-Kit+AA4.1+ population in wt (n = 4), het (n = 12), and ko (n = 6) fetal livers. Bars indicate average number for each group. (C) Number of hematopoietic colonies generated in methylcellulose from wt (n = 4), het (n = 12), and ko (n = 6) fetal livers. Bars indicate average number for each group.
Fetal liver hematopoiesis in distal Runx1-deficient embryos. (A) FACS analysis of AA4.1 and c-Kit expression in E14.5 fetal livers (FL) isolated from distal+/+ (wt), distal+/− (het), and distal−/− (ko) embryos. (B) Percentage of c-Kit+AA4.1+ population in wt (n = 4), het (n = 12), and ko (n = 6) fetal livers. Bars indicate average number for each group. (C) Number of hematopoietic colonies generated in methylcellulose from wt (n = 4), het (n = 12), and ko (n = 6) fetal livers. Bars indicate average number for each group.
Discussion
The transcription factor RUNX1/AML1 is a master regulator of hematopoietic development, and its transcription is under the control of 2 promoters. The significance of these alternative promoters and their relative activities remain largely unknown. In the present study, we have addressed these questions by introducing 2 reporter genes under the control of the proximal and distal promoters.
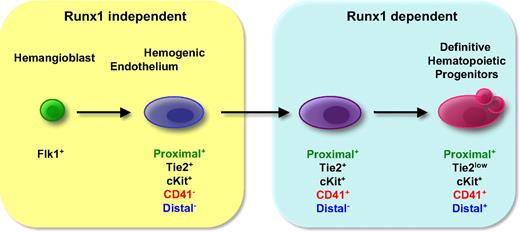
The transcriptional activity of the proximal Runx1 promoter was first detected within the Flk-1+ cell population in EBs and in embryos, whereas the onset of distal Runx1 promoter activity was detected later in CD41+ cells. This sequential activation of the proximal and then distal Runx1 promoter is remarkably distinct from results in zebrafish, where a transgenic P1 promoter was shown to be activated before a transgenic P2 promoter.26 This finding suggests inherent differences in the specific regulation of the 2 Runx1 promoters in the 2 species. We have recently shown that the hemangioblast generates hematopoietic cells through an intermediate hemogenic endothelium, present within a Tie2hic-Kit+CD41− cell population.13 The present study further determines that the subfraction of Tie2hic-Kit+CD41− cells in which the proximal Runx1 promoter is active gives rise to CD41+ cells. In the model of hematopoietic development depicted in Figure 7, we therefore refer to this proximal-hCD4+/Tie2hic-Kit+CD41− cell population as hemogenic endothelial. Subsequently these cells gain CD41 expression to become proximal-hCD4+/Tie2hic-Kit+CD41+. The authors of previous studies have established that RUNX1 is critically required for the acquisition of CD41 by c-Kit+ cells.13,36,43 Our results further suggest that RUNX1 controls the transition from hemogenic endothelium to this subsequent cell population, at a stage when only the transcription from the proximal promoter of Runx1 is detected.
Transcriptional activities of proximal and distal Runx1 promoters during the progression from hemogenic endothelium to hematopoietic progenitors.
Transcriptional activities of proximal and distal Runx1 promoters during the progression from hemogenic endothelium to hematopoietic progenitors.
The proximal-hCD4+/Tie2hic-Kit+CD41+ cells then start to express distal-GFP and begin to lose Tie2 expression. The potential to give rise to mature definitive hematopoietic cells correlates with distal-GFP expression (Figure 1G). This finding indicates that the onset of distal Runx1 expression defines the transition to definitive hematopoietic progenitors. These data suggest that either losing Tie2 expression might trigger the expression of distal Runx1, or, alternatively, that expression from distal promoter and/or greater level of RUNX1 protein may result in Tie2 down-regulation and loss of endothelial phenotype. Further analysis will be needed to obtain evidence for either of the aforementioned hypotheses. The correlation between distal-GFP detection and hematopoietic potential observed upon ES-cell differentiation was further confirmed in vivo in E8.5 to E10.5 embryos (Figure 4). In addition, in fetal liver, we detected hematopoietic potential in both proximal-hCD4+/distal-GFP− and proximal-hCD4+/distal-GFPhigh populations (Figure 5C), but the generation of hematopoietic colonies by the proximal-hCD4+ single-positive cells was associated with gain of distal-GFP expression. Finally, at day 12.5 of gestation, the fetal liver cells expressing hematopoietic markers, such as CD45, AA4.1, and c-Kit, were mainly found in the distal-GFP+ cell population (Figure 5B). Altogether, our data demonstrate that transcriptional activity of the distal promoter of Runx1 marks definitive hematopoietic progenitors at all investigated stages of development, from their appearance in the yolk sac at early sp stages to the fetal liver hematopoiesis.
On the basis of the enrichment of definitive hematopoietic potential in distal-GFP+ population, it seemed likely that the distal isoform of RUNX1 plays an important role in the establishment of definitive hematopoiesis. To address this question, we analyzed embryos lacking distal RUNX1. Surprisingly, hematopoietic development was not affected in these embryos. This observation suggests that the main and critical requirement for Runx1 expression is restricted to the proximal isoform at the hemogenic endothelium stage of development. This finding is in accordance with our recent results demonstrating that a single 12-hour pulse of RUNX1b expression during Runx1−/− blast colony development is sufficient to rescue the development of definitive hematopoietic precursors44 and with the fact that RUNX1 is largely dispensable in adult hematopoiesis when mainly the distal promoter is active. Altogether, these findings suggest that the activity of the proximal promoter is critical for the emergence of hematopoietic cells, whereas the activity of the distal promoter is dispensable in hematopoietic maintenance.
Our data demonstrate that the differential activities of Runx1 promoters define major steps of hematopoietic specification. These findings suggest that the expression of proximal Runx1 in a hemogenic endothelial cell population is the critical step for the emergence of definitive hematopoietic cells. Our reporter ES-cell and mouse lines will facilitate further dissection of the regulation of Runx1 expression. Identification and access to these discrete stages of hematopoietic development will provide the opportunity to further explore the cellular and molecular mechanisms of hematopoietic development.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are grateful to the members of the FACS laboratory for help in cell analysis and cell sorting, to members of the Molecular Biology Core Facility for help in sequencing and genotyping, and to members of the Biological Resource Unit for help in animal maintenance and breeding. We thank members of the laboratory and C. Bonifer for critically reading the manuscript.
Authorship
Contribution: P.S. designed and performed most of the experiments and drafted the manuscript; V.K. and G.L. designed and supervised research; C.L. designed experiments; C.L. and G.L. performed experiments; V.K. helped with writing the manuscript; and G.L. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Georges Lacaud, Stem Cell Biology, Paterson Institute for Cancer Research, University of Manchester, Wilmslow Rd, Manchester M20 4BX, United Kingdom; e-mail: glacaud@picr.man.ac.uk.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal