Whether complement activation plays a role in the therapeutic efficacy of unconjugated therapeutic monoclonal antibodies such as rituximab has been widely debated. In this issue of Blood, Wang and colleagues suggest that complement has an inhibitory effect on NK cell–mediated cytotoxicity in vitro and therapeutic monoclonal antibody efficacy in vivo.
Most unconjugated therapeutic monoclonal antibodies (mAbs) that have entered the clinic for cancer treatment are of the human IgG1 isotype that activates complement and binds to FcγRs on natural killer (NK) cells, macrophages, and neutrophils, thus mediating complement-dependent cytotoxicity (CDC), antibody-dependent cytotoxicity (ADCC), and phagocytosis. The best studied is the chimeric anti-CD20 antibody rituximab, and most evidence suggests that the immune modulating properties of this antibody are responsible for its efficacy in vivo.
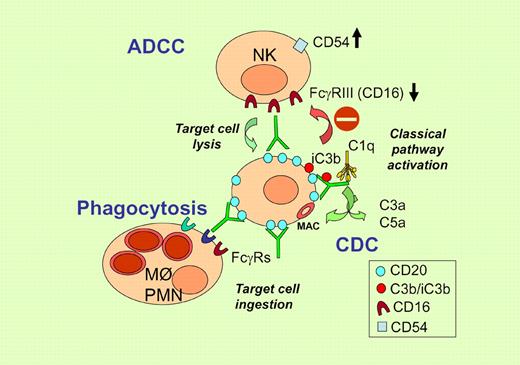
The classical complement pathway is activated by target bound antibodies that bind the C1q complex, which in turn activates the complement cascade. Cleavage of soluble C3 generates the C3b fragment, which covalently attaches to nearby proteins on the cell surface or on the antibody itself. Cleavage of C5 catalyzes formation of the membrane attack complex (MAC), which forms membrane pores leading to cell lysis through osmotic shock. Initial studies demonstrating a high complement-activating capacity of rituximab and efficient lysis of lymphoma cells in vitro suggested that CDC is an important mechanism of action.1 In support of this are several murine models of lymphoma, in which the role of complement was shown using either C1q knockout (KO) animals or C3 depletion in vivo by cobra venom factor (CVF). In contrast, other murine models investigating depletion of normal B cells by CD20 antibodies have indicated no role of complement.2 The reasons for this difference may include target cell type (normal B cells or tumor), CD20 expression levels, mouse strain, tissue localization, and also the actual antibody used in the assay (rituximab or mouse anti-CD20 antibodies).
The work of Wang et al adds another piece to the puzzle by showing that complement activation by mAbs can actually be detrimental to therapeutic activity.3 The same group had previously demonstrated that complement activation by rituximab resulted in decreased ADCC due to C3 deposition onto the target-bound antibody and reduced binding of opsonized targets to FcγR on NK cells,4 as illustrated in the figure. In the report in this issue of Blood, they extend these studies to complement present in extravascular fluids, showing that these fluids, in the form of transudative pleural fluid and nonmalignant ascites, contain sufficient complement activity to lead to C3b deposition onto the cell surface in the presence of rituximab. Furthermore, this complement fixation inhibits NK-cell activation in vitro. They use down-modulation of CD16 and induction of CD54 on NK cells as surrogate markers of ADCC. Inhibition of NK-cell activation is abolished by C3 depletion with CVF or a recombinant analog thereof. Most importantly, this inhibition appears to take place in vivo considering that C3 depletion increases the therapeutic activity of an anti-idiotype antibody in a syngeneic lymphoma model.
Illustration of immune-mediated mechanism of action of rituximab and inhibition of ADCC by complement fragments deposited on the antibody. Increased CD54 and decreased CD16 expression are used as surrogate markers of ADCC.
Illustration of immune-mediated mechanism of action of rituximab and inhibition of ADCC by complement fragments deposited on the antibody. Increased CD54 and decreased CD16 expression are used as surrogate markers of ADCC.
The work of Wang and colleagues is important because it presents the first evidence that complement activation can have a detrimental effect on the antitumor activity of a therapeutic mAb in vivo. It favors the idea of modifying antibodies, for example through point mutations, to inhibit their complement-activating function and thus augment ADCC activity. Several new-generation anti-CD20 antibodies have already been designed, originally aimed at diminishing the acute side effects related to complement activation.2
Clearly, these in vivo findings will have to be extended to other mouse models and different therapeutic mAbs including rituximab, because different murine models have been widely contradictory with respect to the role of complement or immune cells in the activity of anti-CD20 antibodies. In support of this report, in a recent study of B-cell depletion using rituximab in mice transgenic for human CD20, a small inhibitory effect of complement was observed.5 Nonetheless, there are a number of caveats regarding the conclusions reached by Weiner's group.3 One is their use of an intraperitoneal route of tumor inoculation. Previous evidence suggests that normal peritoneal B cells are more resistant to depletion by anti-CD20 and that resistance can be overcome by inducing inflammation at this site.6 Because CVF cleaves C3 in the soluble phase and complement fragments have multiple functions, alternative explanations for the data of Wang et al are possible. CVF treatment in the peritoneum may have attracted or activated effector cells at this site, thus enhancing the antibody effect. Indeed the mechanism of enhancement of therapeutic activity by complement depletion in vivo still needs to be investigated in more detail, verifying the role of different immune cell populations. Another caveat is that Wang et al used anti-CD20 in vitro and anti-idiotype antibody in vivo, which may not have identical mechanisms of action.
Finally, the significance of the findings to the clinic need to be verified. A role of ADCC in patients treated with rituximab is indicated by the correlation between CD16 polymorphism and clinical response of FL patients to rituximab.2 In addition, a C1qA polymorphism associated with low C1q levels correlated with prolonged response of FL patients to rituximab,7 supporting a negative role of complement activation in humans. In contrast to these data, some authors have suggested that coadministration of fresh frozen plasma (a source of complement) with rituximab improves the response of B-CLL patients refractory to the drug.8 Probable light will be shed by the clinical results obtained with new-generation anti-CD20 antibodies that have entered the clinic and have enhanced capacity to induce CDC, ADCC, or programmed cell death.2
Conflict-of-interest disclosure: The authors have received research grants from Roche in the past 5 years. ■