Abstract
A specific splice variant of the CD44 cell- surface protein family, CD44v6, has been shown to act as a coreceptor for the receptor tyrosine kinase c-Met on epithelial cells. Here we show that also on endothelial cells (ECs), the activity of c-Met is dependent on CD44v6. Furthermore, another receptor tyrosine kinase, VEGFR-2, is also regulated by CD44v6. The CD44v6 ectodomain and a small peptide mimicking a specific extracellular motif of CD44v6 or a CD44v6-specific antibody prevent CD44v6-mediated receptor activation. This indicates that the extracellular part of CD44v6 is required for interaction with c-Met or VEGFR-2. In the cytoplasm, signaling by activated c-Met and VEGFR-2 requires association of the CD44 carboxy-terminus with ezrin that couples CD44v6 to the cytoskeleton. CD44v6 controls EC migration, sprouting, and tubule formation induced by hepatocyte growth factor (HGF) or VEGF-A. In vivo the development of blood vessels from grafted EC spheroids and angiogenesis in tumors is impaired by CD44v6 blocking reagents, suggesting that the coreceptor function of CD44v6 for c-Met and VEGFR-2 is a promising target to block angiogenesis in pathologic conditions.
Introduction
Angiogenesis is a complex process that leads to the formation of new blood vessels from existing ones. During embryogenesis, angiogenesis complements vasculogenesis, the production of new blood vessels from hematopoietic precursors. In the adult organism, angiogenesis takes place under normal conditions during the female reproductive cycle or under pathologic conditions, such as in tumor growth and wound healing. Secretion of angiogenic factors from the tumor mass induces the formation of blood vessels, which feed cancer cells with oxygen and nutrients. These vessels will eventually be used as a route for the spreading of metastases.1
Several angiogenic factors have been described, including vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), transforming growth factor-α (TGF-α), TGF-β, hepatocyte growth factor (HGF), tumor necrosis factor, angiogenin, interleukin-8, and the angiopoietins.2 The most prominent angiogenic factor is VEGF-A, a member of the VEGF family of growth factors also including placental growth factor and VEGF-B, -C, -D, and -E.3 VEGFs bind to 3 related members of the VEGFR family, VEGFR-1, -2, and -3. The importance of VEGFs and their receptors is demonstrated by the phenotypes of the respective knockout mice. Indeed, Vegf-A and Vegfr-2 knockout mice show a failure in vasculature formation and die during embryogenesis, whereas Vegfr-1–deficient mice die of an overgrowth of blood vessels.3
Fighting angiogenesis has become an attractive aim of cancer therapy. Indeed, targeting angiogenesis rather than directly addressing the tumor cells has the advantage that the same reagents can be applied to many different types of tumors. In addition, because of the low turnover rate of endothelial cells (ECs), they are less susceptible to become resistant to chemotherapy.4
Several anti-VEGF treatment regimens already exist that can be combined with chemotherapy or radiotherapy. These treatments make use of VEGF inhibitors, such as antibodies against VEGF (bevacizumab), several small molecules inhibiting VEGFR-2 signaling, as well as soluble VEGF receptors that compete with the endogenous receptor for binding to VEGF.5 However, because all these treatments have a relatively modest benefit for most cancer patients, there is still plenty of room for improvement.
HGF is another potent angiogenic factor: the expression of HGF and its receptor c-Met correlates with tumor vascularization,6 the production of VEGF in a variety of cells and tissues is induced by HGF,7 and HGF can potentiate the activity of VEGF.8-10 Furthermore, HGF leads to mobilization of endothelial progenitor cells,11 and the expression of a soluble c-Met receptor (decoy Met) impairs tumor angiogenesis.12
We have previously shown that HGF depends on a CD44 exon v6 containing isoform for the activation of c-Met on epithelial cells.13 CD44 isoforms containing the variant exon v6 have been shown to be metastatic determinants.14 The role of CD44v6 in metastasis results most probably from its cooperation with the receptor tyrosine kinase (RTK) c-Met. In many carcinoma and primary cells, the activation and signaling from the c-Met receptor can be blocked by CD44v6-specific antibodies, by CD44v6 siRNA13 and, most interestingly, by CD44 exon v6-specific peptides.15 CD44v6 isoforms play a dual role for c-Met-dependent signaling. Their extracellular part is required for c-Met activation, whereas the cytoplasmic domain of CD44 recruits ERM proteins (Ezrin-Radixin-Moesin) that bind the cytoskeleton and promote activation of Ras by its guanidine exchange factor (GEF) son of sevenless (SOS).16
Because HGF and c-Met are also important in angiogenesis and because angiogenesis is impaired in CD44 null mice,17 we have examined whether HGF, c-Met, and the CD44v6 isoform also cooperate in ECs. A CD44v6-specific peptide as well as a CD44v6 antibody can indeed block c-Met activation on ECs and HGF-induced migration and tubular vessel formation by ECs is impaired. Most interestingly, this peptide and the antibody also blocked VEGF induced VEGFR-2 activation. In addition, ERM proteins that associate with CD44 regulate signaling from VEGFR-2. Therefore, the mechanism of action of CD44 seems very similar for both c-Met and VEGFR-2 activation. Indeed, CD44v6 controls physiologic changes, including the formation of blood vessels in vitro and in vivo and most importantly angiogenesis in a tumor.
Methods
Cells and cell culture
Human umbilical vein endothelial cells (HUVECs; Provitro GmbH) were grown in Endothelial Cell Growth Medium complemented with the Supplement Mix (Provitro GmbH). The human aortic ECs (HAOECs, Promocell) and human cardiac microvascular ECs (HCMECs, Promocell) were grown in Endothelial Cell Growth Medium MV (Promocell) complemented with the supplements according to the manufacturer's instructions. All ECs were passaged less than 10 times. The HEK293 cells (ATCC) and the human breast cancer cells MDA-MB231 (ATCC) were grown in Dulbecco modified Eagle medium (Invitrogen) supplemented with 10% fetal calf serum (FCS; PAA Laboratories). The rat pancreatic carcinoma cells BSp73AS and its transfectant BSp73ASs613 were grown in RMPI (Invitrogen) plus 10% FCS. The human pancreatic cancer cells L3.6pl,18 kindly provided by C. Bruns, University of Munich, Germany, were maintained in Dulbecco modified Eagle medium supplemented with 10% FCS, sodium pyruvate, nonessential amino acids, l-glutamine, and a 2-fold vitamin solution (Invitrogen).
Antibodies and other reagents
The human monoclonal antibody against CD44v6 (VFF18) was from Bender, the pan-CD44 antibody IM7 from BD Biosciences, the anti-Erk 1 (K-23) antibody from Santa Cruz Biotechnology, and the phospho-VEGFR-2 Tyr1175 and the phospho-Erk phospho-p44/42 from Cell Signaling Technology. The antibody against VEGFR-2 was from R&D Systems and secondary antibodies labeled with horseradish peroxidase were from Dako. The murine CD44v6 antibody has been described (clone 9A4,19 ). VEGF-A165 and VEGF-A121 were produced in Pichia pastoris as described.20 HGF was a generous gift of George Vande Woude (Van Andel Institute). The CD44v6 human peptide (14mer) and the control peptide have been described.15 The sequence of the v6 murine peptide is: QETWFQNGWQGKNP.
Constructs and protein production
The VEGFR-2 expression plasmid pBE hVEGFR-2 was derived from the pEGFP-C1 vector from Clontech by introducing the sequence encoding the human VEGFR-2 with the PCR subcloning method to remove the GFP reading frame21 with the forward primer: GCTCTTCGGGGAGCAGCGATGGAGAGCAAGGTGCTGCTG and the reverse primer: GGAGGTTTTTTAAAGCAAGTAAAACCTTTATCACAGATCCTCTTCTGAGATGAG.
The constructs encoding the soluble wild-type and mutant GST-CD44 cytoplasmic domain have been described22 and were obtained from C. Isacke (Breakthrough Breast Cancer Research Center). The EzrinΔABD expressing construct23 was a gift from M. Arpin (Institute Pasteur).
The expression vectors for producing VEGF-A and the CD44v6 ectodomain (CD44v6ECD and CD44v6ECD mut) in P pastoris were generated using the pPICZαA vector system from Invitrogen as described.20 All expression vectors were made with the PCR subcloning technology, and all recombinant proteins except for VEGF-A165 carry a hexahistidine tag at the amino terminus. P pastoris strain X33 was transfected by electroporation, and Zeocin-resistant clones were picked and tested for transgene expression on methanol induction. Secreted proteins were purified from the yeast culture supernatant by immobilized metal affinity chromatography and polished on Superdex 200 (GE Healthcare).
Transfection
HEK293 cells were transiently transfected with Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol in a 6-well plate. Transfection of the BSp73AS cells and BSp73ASs6 cells was performed by electroporation. In brief, 3 × 106 cells were mixed with 5 μg of vector DNA on ice; the electroporation was performed in a 4-mm electroporation cuvette using a Gene Pulser (Bio-Rad) at 250 μF, 0.28 kV. Prewarmed medium containing serum was added, and the cells were distributed in a 6-well plate. The cells were then grown for 24 hours before the experiment.
Activation of RTKs and Erk
Serum-starved cells (24 hours) were induced with the growth factor HGF (20 ng/mL), platelet-derived growth factor (PDGF; 20 ng/mL) at 37°C for 5 minutes or with VEGF-A165 or VEGF-A121 (40 ng/mL) at 37°C for 8 minutes. Where indicated, the cells were treated with blocking reagents before induction at 37°C for 10 minutes (100 μg/mL anti-CD44v6, 100 ng/mL CD44v6 peptide or control peptide, 0.5 μg/mL CD44v6ECD). Cells were washed with ice-cold phosphate-buffered saline (PBS). To detect activated Erk, cells were lysed in boiling sodium dodecyl sulfate (SDS)–sample buffer containing 100mM dithiothreitol (DTT) and subjected to Western blot analysis using antibodies against phosphorylated Erk. The Erk loading control was performed on the same blot, stripped (62.5mM Tris, pH 6.8, 2% SDS, 0.8% DTT), and probed with the Erk antibody.
To detect activated VEGFR-2 or c-Met, cells were lysed in reducing sample buffer and the blots of SDS–polyacrylamide gel electrophoresis gels were probed with an antibody against phosphorylated VEGFR-2 or c-Met. Alternatively cells were lysed in 20mM Tris pH 7.4, 1mM ethylenediaminetetraacetic acid, 1mM ethyleneglycoltetraacetic acid, 1mM DTT, 25mM NaCl, 1.5% Triton X-100, 10mM NaF, 1mM phenylmethylsulfonyl fluoride, 1mM Na-orthovanadate, 1mM aprotinin and 1mM leupeptin. After centrifugation, the cleared lysates (10 000g for 15 minutes) were incubated with a VEGFR-2 antibody or a mouse IgG control at 4°C overnight followed by incubation with protein A/G agarose beads (Merck) for 2 hours at 4°C. The beads were washed 3 times, boiled in sample buffer, and subjected to Western blot analysis using phospho-specific VEGFR-2 antibody. For the loading control, the blots were stripped and reprobed with the respective antibody. Blots were stained using the enhanced chemiluminescence system (Thermo Fisher Scientific). Bands in Western blot analysis were quantified with the program ImageJ (National Institutes of Health).
Coimmunoprecipitation
For coimmunoprecipitation HUVECs (1.5 × 106 in 10-cm plates) were induced by the respective ligands as indicated. The cells were incubated in lysis buffer (25mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, pH 7.5, 100mM NaCl, 10mM MgCl2, 1mM ethylenediaminetetraacetic acid, 10% glycerol, 1% Igepal, 10mM NaF, 1mM phenylmethylsulfonyl fluoride, 1mM Na-orthovanadate, and 1mM aprotinin and 1mM leupeptin) for 30 minutes on ice and then centrifuged for 20 minutes at 12 000g. The cleared lysates were incubated with antibody at 4°C overnight and then precipitated with protein A/G agarose beads (Merck). The precipitates were washed 3 times in lysis buffer and subjected to Western blot analysis.
Scratch assay
HAOECs or HUVECs were seeded in 12-well plates at a concentration of 2.5 × 105 cells per well. After 24 hours, a scratch was made into the confluent cell layer using a sterile pipette tip. The medium was changed and replaced by fresh medium or a medium containing 100 ng/mL v6 peptide or 100 ng/mL control peptide. After 10 minutes at 37°C, induction with the growth factors (HGF, 20 ng/mL; VEGF-A165, 40 ng/mL; or VEGF-A121, 40 ng/mL) was performed. Pictures of the cells (original magnification, ×100) were taken 24 hours after induction using a Canon Power Shot S620 digital camera. The computer program ImageJ was used for quantitative evaluation. The area covered by cells in the scratch was quantified.
Sprouting assay
Spheroids of HUVECs (750 cells) were generated in hanging drops as described.24 They were suspended in Endothelial Cell Growth Medium (Promocell) containing 1 mg/mL rat tail collagen I (BD Biosciences) and 0.6% (wt/vol) methylcellulose and distributed in 48-well plates (30 spheroids/well). After solidification at 37°C, the mixture was overlaid with Endothelial Cell Growth Medium (Promocell) containing 100 ng/mL v6 peptide or control peptide and VEGFs (40 ng/mL). Pictures (original magnification, ×100) were taken 48 hours later using a Canon Power Shot S620 digital camera and sprouting was quantified with the computer program ImageJ.
Tubule formation
Forty-eight-well plates were coated with growth factor reduced Matrigel (BD Biosciences) mixed with Endothelial Cell Growth Medium (Promocell) in a ratio 1:1; 2.5 × 104 HUVECs were seeded per well. CD44v6 peptide or control peptide (100 ng/mL) was added for 10 minutes at 37°C followed by the induction with growth factors. Pictures (original magnification, ×50) were taken 24 hours later using a Canon Power Shot S620 digital camera.
In vivo angiogenesis assay
All animals were handled according to German regulations for animal experimentation. The animal experiments were approved by the Regierungspäsidium Karlsruhe (35-9185.81/G-83/04). All mice were obtained from Harlan. A spheroid based in vivo angiogenic assay was performed as described.24 Spheroids of HUVECs (100 cells) were generated (“Sprouting assay”), collected by gentle centrifugation (5 minutes, 800g), washed with Endothelial Cell Growth Medium, and mixed with 600 μL of growth factor reduced Matrigel (BD Biosciences), fibrinogen (2 mg/mL; Calbiochem) together with HGF or VEGF-A165 (500 ng/mL), 20 μg of peptide, and thrombin (0.4 U; Calbiochem). On subcutaneous implantation, every second day the peptides (20 μg per mouse) were injected close to the Matrigel/fibrin plugs. Mice were killed 21 days after implantation.
Immunohistologic analysis
After fixation in formalin, Matrigel/fibrin plugs (see Figure 7A) or tumor tissues (Figure 7B) were processed and embedded in paraffin; 7-μm sections of paraffin blocks were deparaffinized and rehydrated.
Matrigel/fibrin plug sections were stained with a mouse-antihuman CD34 antibody (clone QBEND/10, 20 μg/mL, 2 hours; Novocastra) after blocking with 10% goat serum (Dako) for 60 minutes. The sections were then incubated with the goat anti–mouse AlexaFluor 488 (Invitrogen) for 45 minutes. Nuclei were stained with the Hoechst dye 33258 (Sigma-Aldrich). Images of the Matrigel/fibrin area were taken using an OlympusIX50 inverted microscope. Fluorescent structures in the complete matrix area were counted.
In the tumor sections, endogenous peroxidases were blocked with 3% H2O2 in PBS followed by incubation with avidin/biotin (Dako). Unspecific binding was blocked with 10% rabbit serum (BD Biosciences) for 60 minutes followed by incubation with the rat anti–mouse CD31 antibody (0.5 μg/mL overnight at 4°C). Sections were then incubated with a biotinylated rabbit anti–rat antibody (2 μg/mL, 45 minutes) followed by a streptavidin-peroxidase conjugate (Dako) treatment and developed with DAB substrate system (3,3′-diaminobenzidine; Biozol).
Quantification and statistical analysis
All quantifications are given as mean plus or minus SD. Differences between the various conditions were analyzed by paired Student t test, and P less than .05 was considered as statistically significant.
Results
CD44v6 controls activation of VEGFR-2 and downstream signaling
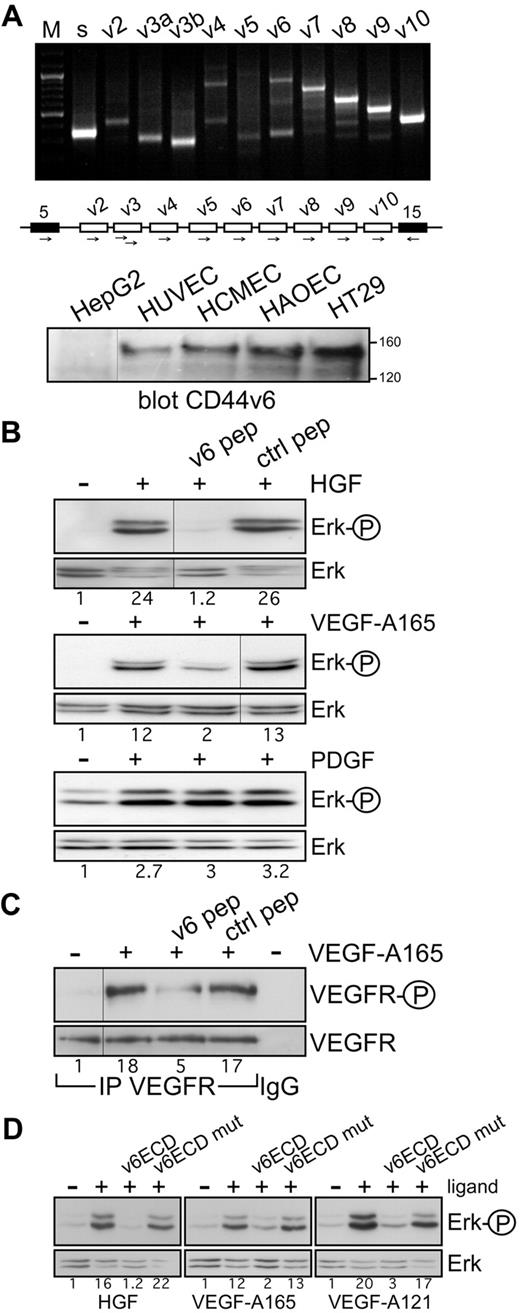
In a variety of cancer cell lines and primary cells, c-Met activation and signaling can be blocked by CD44v6 antibodies and peptides.13,15 To test whether a CD44 isoform could also act as a coreceptor for c-Met in ECs, we first examined the expression profile of CD44 variants in HUVECs by exon-specific RT-PCR analysis25 (Figure 1A). Several variant isoforms are indeed expressed. Interestingly, exon v6 seems to be expressed together with exon v7-10 (indicated by the ladder) and probably also alone (indicated by the lower band in the v6 lane). Note that v6 seems not to be coexpressed with exon v3 that appears as an independent isoform. In addition, on the protein level, CD44v6-containing isoforms were detected in HUVECs (Figure 1A).
Coreceptor function of CD44v6 in ECs. (A) CD44 variant exon-specific RT-PCR analysis in HUVECs. The method and the primers have been described.13,25 “s” refers to the use of 2 primers in the CD44 constant exons 5 and 15 (black boxes in the schematic drawing of the relevant parts of the CD44 gene); the other lanes refer to PCRs performed with the forward primers in variant exons and the reverse primer in exon 15. “M” refers to a DNA ladder. The Western blot shows a staining of cell lysates with the CD44v6-specific antibody VFF18. A total of 20 μg of protein was applied to each, except for HT29 (5 μg). Size markers for apparent molecular weight are indicated. HepG2 were used as CD44v6 negative cells, HT29 as positive ones. (B) Signal transduction induced by HGF, VEGF-A165, or PDGF in untreated HUVECs or HUVECs treated with the human v6-specific 14mer peptide or a control peptide. Activation of Erk was measured as described in “Activation of RTKs and Erk.” (C) VEGFR-2 activation by VEGF-A165 in HUVECs was determined after immunoprecipitation of VEGFR-2 and Western blotting with the phospho-specific VEGFR-2 antibody. IgG indicates a control precipitation. Treatment with VEGF-A165 and with the peptides was performed as described in “Activation of RTKs and Erk.” (D) Ligand-induced signaling in HUVECs in the presence of the CD44v6ECD or a mutated version as indicated. Treatments were done as described in “Activation of RTKs and Erk.” The numbers indicate the fold induction as calculated by the computer program ImageJ. All experiments were performed at least 3 times and gave similar results. Vertical lines have been inserted to indicate repositioned gel lanes.
Coreceptor function of CD44v6 in ECs. (A) CD44 variant exon-specific RT-PCR analysis in HUVECs. The method and the primers have been described.13,25 “s” refers to the use of 2 primers in the CD44 constant exons 5 and 15 (black boxes in the schematic drawing of the relevant parts of the CD44 gene); the other lanes refer to PCRs performed with the forward primers in variant exons and the reverse primer in exon 15. “M” refers to a DNA ladder. The Western blot shows a staining of cell lysates with the CD44v6-specific antibody VFF18. A total of 20 μg of protein was applied to each, except for HT29 (5 μg). Size markers for apparent molecular weight are indicated. HepG2 were used as CD44v6 negative cells, HT29 as positive ones. (B) Signal transduction induced by HGF, VEGF-A165, or PDGF in untreated HUVECs or HUVECs treated with the human v6-specific 14mer peptide or a control peptide. Activation of Erk was measured as described in “Activation of RTKs and Erk.” (C) VEGFR-2 activation by VEGF-A165 in HUVECs was determined after immunoprecipitation of VEGFR-2 and Western blotting with the phospho-specific VEGFR-2 antibody. IgG indicates a control precipitation. Treatment with VEGF-A165 and with the peptides was performed as described in “Activation of RTKs and Erk.” (D) Ligand-induced signaling in HUVECs in the presence of the CD44v6ECD or a mutated version as indicated. Treatments were done as described in “Activation of RTKs and Erk.” The numbers indicate the fold induction as calculated by the computer program ImageJ. All experiments were performed at least 3 times and gave similar results. Vertical lines have been inserted to indicate repositioned gel lanes.
c-Met can be activated in HUVECs; and indeed, the HGF-induced activation of Erk can be completely abrogated by a CD44v6 peptide (Figure 1B). This result suggests that in ECs, similarly to epithelial cells, CD44v6 isoforms act as coreceptors for c-Met.
We also tested the effect of the CD44v6 peptide on the activation of VEGFR-2, the most prominent RTK involved in angiogenesis. We activated VEGFR-2 with VEGF-A165, the predominant isoform of the VEGF family.3 Most interestingly, the v6-specific peptide abrogated activation of VEGFR-2 and downstream Erk activation in HUVECs (Figure 1B-C). PDGF-dependent activation, however, was not inhibited by the v6 peptide, in agreement with previous results obtained with epithelial cells,15 demonstrating the specificity of action of the CD44v6 peptide. To further confirm the dependency of VEGFR-2 and c-Met on CD44v6 in ECs, we tested the effect of a soluble CD44v6 ectodomain (CD44v6ECD) on their activation. The CD44v6ECD completely abrogated the activation of Erk induced by HGF and VEGF-A165 (Figure 1D). In contrast, a CD44v6ECD mutated in the 3 amino acids instrumental for the coreceptor function of CD44v6 for c-Met15 did not have any effect (Figure 1D).
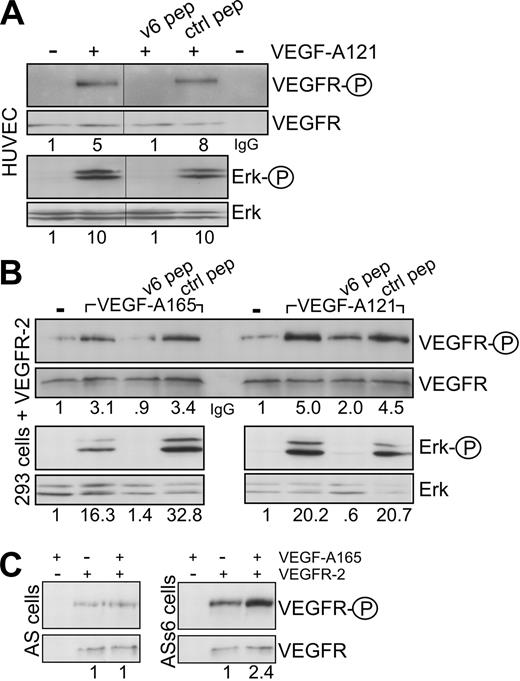
CD44 proteins containing the exon v3 can be modified by heparan sulfate (HS). This modification seems to be required to bind growth factors, such as FGF or HB-EGF.26 Binding of VEGF-A165 to HS proteoglycans and to HS-modified CD44 isoforms has also been described.27 The RT-PCR analysis in HUVECs, however, suggests that exon v6 and exon v3 are not coexpressed on the same isoform; thus, the activation by VEGF-A165 might be independent on HS modification. To test the requirement of HS residues for the VEGFR-2 activation, we treated the HUVECs with VEGF-A121 that lacks exons 6 and 7. Exon 7 accounts for binding to HS.28 VEGF-A121 was also able to activate VEGFR-2 and Erk in HUVECs (Figures 1D, 2A), and this activation was again completely blocked by the CD44v6ECD (Figure 1D) and a CD44v6 peptide (Figure 2A). In addition, in HEK293 cells transfected with a VEGFR-2 expression vector, the CD44v6 peptide blocked activation of VEGFR-2 induced by VEGF-A165 or VEGF-A121 (Figure 2B), suggesting that activation of VEGFR-2 occurs independently of HS modifications. To confirm that activation of VEGFR-2 was indeed independent of HS modification of CD44, we tested whether a CD44 variant isoform containing exclusively the exon v6, as in the case of c-Met,13 was sufficient for VEGFR-2 activation. VEGFR-2 expression vectors were transiently transfected either into rat BSp73AS pancreatic carcinoma cells that express only CD44s or into BSp73AS cells stably transfected with CD44v6 (BSp73ASs613 ). On treatment with VEGF-A165, only the BSp73ASs6 cells were inducible (Figure 2C). Thus, a CD44 variant isoform containing only the variant exon v6 that cannot bind HS was sufficient to act as a VEGFR-2 coreceptor.
The coreceptor function of CD44v6 is independent of heparin sulfation. (A) HUVECs were induced with VEGF-A121 in the presence of the CD44v6-specific peptide or control peptide as indicated. The activation of VEGFR-2 (as in Figure 1C) and Erk was determined. The IgG lane corresponds to an immunoprecipitation with the IgG control antibody. (B) HEK293 cells were transiently transfected with a VEGFR-2 expression construct (see “Transfection”). They were then treated with VEGF and peptides as indicated, and the activation of VEGFR-2 or signaling to Erk was measured directly by Western blotting. (C) BSp73AS cells (AS) or BSp73ASs6 (ASs6) cells were transiently transfected with a VEGFR-2 expression construct and activated with VEGF-A165 where indicated. Activation of VEGFR-2 was determined using the phospho VEGFR-2–specific antibody (Tyr 1175) directly in Western blotting. The numbers refer to fold induction. Vertical lines have been inserted to indicate repositioned gel lanes.
The coreceptor function of CD44v6 is independent of heparin sulfation. (A) HUVECs were induced with VEGF-A121 in the presence of the CD44v6-specific peptide or control peptide as indicated. The activation of VEGFR-2 (as in Figure 1C) and Erk was determined. The IgG lane corresponds to an immunoprecipitation with the IgG control antibody. (B) HEK293 cells were transiently transfected with a VEGFR-2 expression construct (see “Transfection”). They were then treated with VEGF and peptides as indicated, and the activation of VEGFR-2 or signaling to Erk was measured directly by Western blotting. (C) BSp73AS cells (AS) or BSp73ASs6 (ASs6) cells were transiently transfected with a VEGFR-2 expression construct and activated with VEGF-A165 where indicated. Activation of VEGFR-2 was determined using the phospho VEGFR-2–specific antibody (Tyr 1175) directly in Western blotting. The numbers refer to fold induction. Vertical lines have been inserted to indicate repositioned gel lanes.
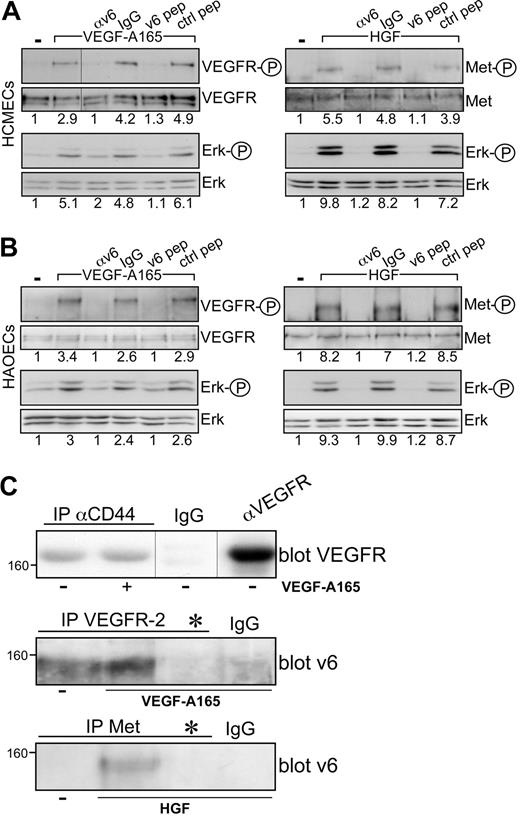
The coreceptor function of CD44v6 for VEGFR-2 can be observed in several cell types, such as HUVECs or HEK293 and BSp73ASs6 transfected with VEGFR-2. The HCMECs and the HAOECs are primary ECs where this collaboration between CD44v6 and c-Met or VEGFR-2 can also be demonstrated (Figure 3A-B). Both cells express CD44v6 proteins (Figure 1A), and VEGF-A165 activation and signaling to Erk are inhibited on treatment of the cells with the CD44v6 peptide and with a CD44v6 antibody (Figure 3A-B).
VEGFR-2 and CD44v6 form a complex. Signal transduction induced by HGF or VEGF-A165 in HCMECs (A) and in HAOECs (B) in the presence of a CD44v6-specific antibody (αv6), IgG control, or the human v6-specific 14mer peptide or a control peptide.15 Activation of VEGFR and Erk was measured as described in “Activation of RTKs and Erk.” Met activation has been described previously.15 (C) Immunoprecipitation with antibodies as indicated and described in “Coimmunoprecipitation” and Western blotting of the precipitates. *Treatment with the CD44v6 peptide (100 ng/mL) before immunoprecipitation with VEGFR-2 or Met antibody to demonstrate the specificity of the CD44v6 band in the Western blot. Size marker for apparent molecular weight is indicated. Vertical lines have been inserted to indicate repositioned gel lanes.
VEGFR-2 and CD44v6 form a complex. Signal transduction induced by HGF or VEGF-A165 in HCMECs (A) and in HAOECs (B) in the presence of a CD44v6-specific antibody (αv6), IgG control, or the human v6-specific 14mer peptide or a control peptide.15 Activation of VEGFR and Erk was measured as described in “Activation of RTKs and Erk.” Met activation has been described previously.15 (C) Immunoprecipitation with antibodies as indicated and described in “Coimmunoprecipitation” and Western blotting of the precipitates. *Treatment with the CD44v6 peptide (100 ng/mL) before immunoprecipitation with VEGFR-2 or Met antibody to demonstrate the specificity of the CD44v6 band in the Western blot. Size marker for apparent molecular weight is indicated. Vertical lines have been inserted to indicate repositioned gel lanes.
Cooperation among CD44 and VEGFR-2 implies that these proteins are in close proximity. Coprecipitation of endogenous CD44v6 and VEGFR-2 from HUVECs confirms this assumption (Figure 3C). Interestingly, the association between these 2 molecules appears to be constitutive, independent of VEGF treatment. This is in contrast to the CD44v6/c-Met association that is HGF-inducible (Figure 3C).13
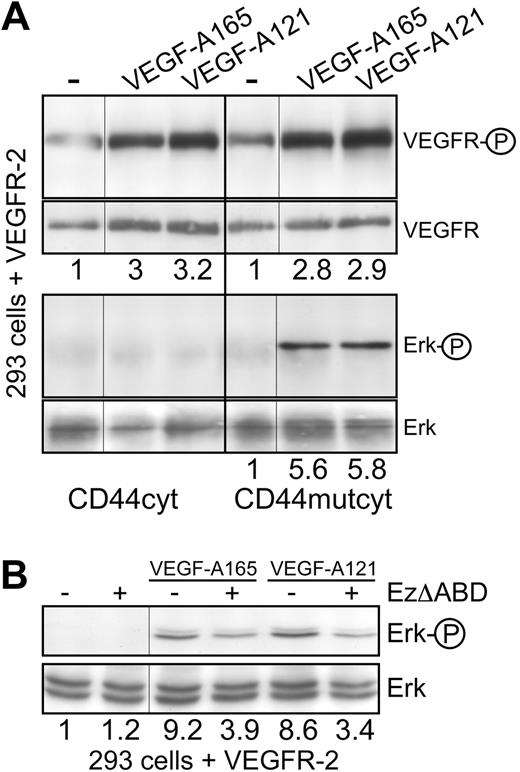
In the case of the c-Met receptor, the CD44 coreceptor associates with ERM proteins and the cytoskeleton to promote signaling. CD44v6, c-Met, HGF, ERM proteins, and the cytoskeleton form a signalosome that allows activation of Ras by its GEF SOS.13,16 To test whether this mechanism also plays a role in the case of VEGFR-2, HEK293 cells were cotransfected with VEGFR-2 and a CD44 cytoplasmic domain-expressing vector to determine whether this domain (CD44cyt) would compete with the activity of endogenous CD44. This was indeed the case. In the presence of CD44cyt, VEGFR-2 signaling to Erk was blocked without affecting VEGFR-2 phosphorylation itself (Figure 4A). Expression of a CD44cyt mutated in the ERM binding sequence22 had no effect on Erk and VEGFR-2 activation (Figure 4A). From these experiments, we conclude that the activation of VEGFR-2 is independent of the cytoplasmic domain of CD44, whereas signal transduction requires this domain and the binding of ERM proteins.
VEGFR-2 signaling is dependent on Ezrin binding to CD44. (A) HEK293 cells transiently transfected with a VEGFR-2 expression construct were cotransfected with vectors expressing either the CD44 cytoplasmic domain (CD44cyt) or the CD44 cytoplasmic domain mutated in the Ezrin-binding site22 (CD44mutcyt). Activation of VEGFR-2 on induction with VEGF (as described in Figure 2C) and signaling to Erk were determined. (B) HEK293 cells transiently transfected with a VEGFR-2 expression construct were cotransfected with vectors expressing EzrinΔABD as indicated, and signaling to Erk on VEGF treatment was determined. The numbers indicate fold induction. Vertical lines have been inserted to indicate repositioned gel lanes.
VEGFR-2 signaling is dependent on Ezrin binding to CD44. (A) HEK293 cells transiently transfected with a VEGFR-2 expression construct were cotransfected with vectors expressing either the CD44 cytoplasmic domain (CD44cyt) or the CD44 cytoplasmic domain mutated in the Ezrin-binding site22 (CD44mutcyt). Activation of VEGFR-2 on induction with VEGF (as described in Figure 2C) and signaling to Erk were determined. (B) HEK293 cells transiently transfected with a VEGFR-2 expression construct were cotransfected with vectors expressing EzrinΔABD as indicated, and signaling to Erk on VEGF treatment was determined. The numbers indicate fold induction. Vertical lines have been inserted to indicate repositioned gel lanes.
To directly address the involvement of ERM proteins, we transfected HEK293 cells with VEGFR-2 together with an ezrin construct lacking the actin-binding domain23 (EzΔABD, Figure 4B). This truncated version of ezrin also inhibited VEGF-A165 and VEGF-A121 signaling to Erk, indicating that the binding of ERM proteins to the cytoskeleton is required for signaling from VEGFR-2.
In conclusion, CD44v6 is a coreceptor for VEGFR-2 in ECs. A CD44v6 antibody, a peptide, and the CD44v6ECD block VEGFR-2 activation. CD44v6 and VEGFR-2 form a constitutive complex, as demonstrated by coimmunoprecipitation experiments. In addition, the cytoplasmic domain of CD44v6 recruits ERM proteins and the cytoskeleton to promote signaling.
A CD44v6 peptide and antibody block the response of ECs to VEGF
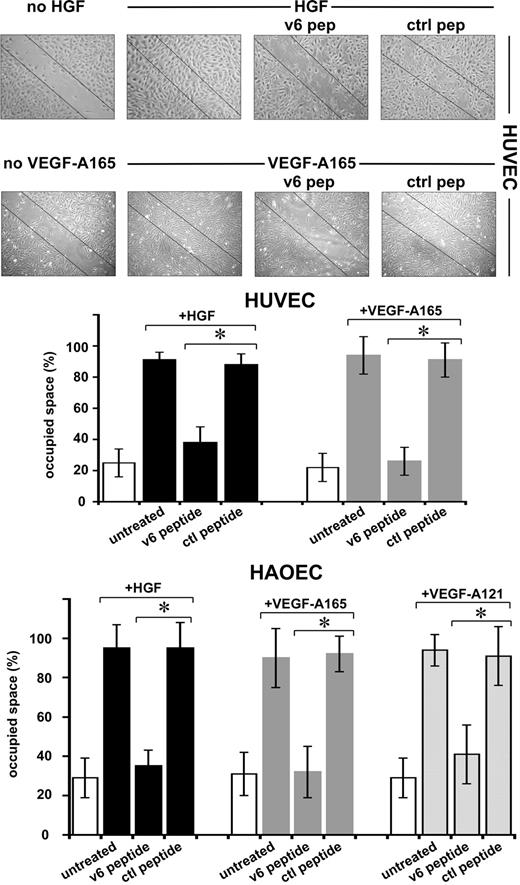
The angiogenic process comprises several steps, ultimately leading to the formation of new capillaries. Several of these steps can be mimicked in vitro. In a scratch assay, migration of ECs can be measured. HGF and VEGF-A induced migration of HUVECs and HAOECs, leading to closure of a scratch in a confluent monolayer (Figure 5). In the presence of the v6 peptide, this process was strongly inhibited, whereas a control peptide had no effect. Measurement of proliferation using a 3H-thymidine incorporation assay revealed that the cells are not proliferating during the time of the observation (not shown). Staining of the cells with trypan blue revealed that the peptide treatment had no toxic effect on the cells (not shown).
Migration of ECs requires CD44v6. An example of a scratch assay (see “Scratch assay”) with HUVECs and the quantification with HUVECs and HAOECs treated with growth factors and peptides as indicated are shown. SD was calculated from 3 independent experiments. *P < .05.
Migration of ECs requires CD44v6. An example of a scratch assay (see “Scratch assay”) with HUVECs and the quantification with HUVECs and HAOECs treated with growth factors and peptides as indicated are shown. SD was calculated from 3 independent experiments. *P < .05.
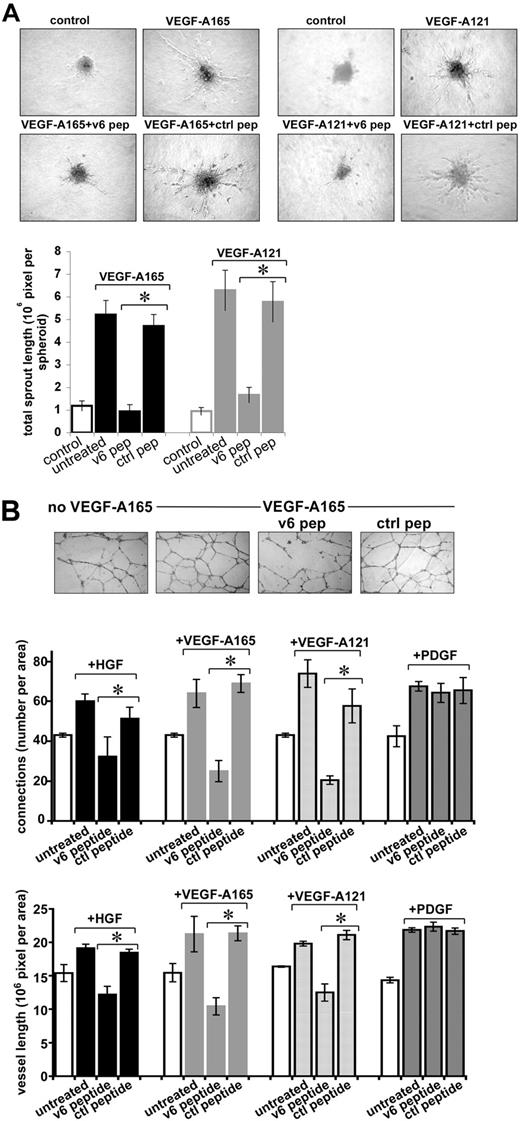
ECs have the property to form spheroids when grown in methylcellulose.29 Spheroids from HUVECs, transferred to collagen in the presence of VEGF-A165 and VEGF-A121, sprout (Figure 6A). Sprouting was strongly inhibited by the CD44v6 peptide but not by a control peptide (Figure 6A).
Sprouting of endothelial spheroids and tube formation of HUVECs depend on CD44v6. (A) Sprouting from spheroids of HUVECs was measured as described in “Sprouting assay.” Growth factor and peptide treatments are indicated. Pictures were taken after 48 hours, and sprouting was quantified using the computer program ImageJ. (B) Tubule formation induced by growth factors and peptides as indicated was quantified by counting the branching points or the total vessel length per field using the computer program ImageJ. An example for VEGF-A165 is shown. *Statistical significance (P < .05). The SD was calculated from 3 independent experiments.
Sprouting of endothelial spheroids and tube formation of HUVECs depend on CD44v6. (A) Sprouting from spheroids of HUVECs was measured as described in “Sprouting assay.” Growth factor and peptide treatments are indicated. Pictures were taken after 48 hours, and sprouting was quantified using the computer program ImageJ. (B) Tubule formation induced by growth factors and peptides as indicated was quantified by counting the branching points or the total vessel length per field using the computer program ImageJ. An example for VEGF-A165 is shown. *Statistical significance (P < .05). The SD was calculated from 3 independent experiments.
Finally, we used a tubular network formation assay to test the role of CD44v6 in the establishment of new blood vessels. HUVECs were grown on growth factor-reduced Matrigel and treated with HGF, VEGF-A165, or VEGF-A121 in the presence of a v6 peptide or a control peptide. In the absence of exogenous growth factors, a preliminary network can be observed (Figure 6B), probably because of traces of growth factors in the Matrigel. Induction with the different growth factors increased the density of the network. Treatment with the CD44v6 peptide interfered with the formation of the network, whereas a control peptide had no effect. Quantification of this assay revealed that the number of branching points was drastically reduced to 50% for HGF and to 30% for VEGF-A165 and VEGF-A121 (Figure 6B). The length of the vessels was also decreased to 60% for HGF and to approximately 45% for VEGF-A165 and VEGF-A121. The formation of the tubular network induced by PDGF was not affected by the v6 peptide, demonstrating its specificity and ruling out that the peptide has a more general effect on angiogenesis.
In conclusion, various assays, such as scratch closure, spheroid sprouting, and tubular network formation, demonstrate that the response of ECs to HGF, VEGF-A165, and VEGF-A121 requires CD44v6.
In vivo development of blood vessels from grafted EC spheroids is impaired on treatment with a CD44v6 peptide
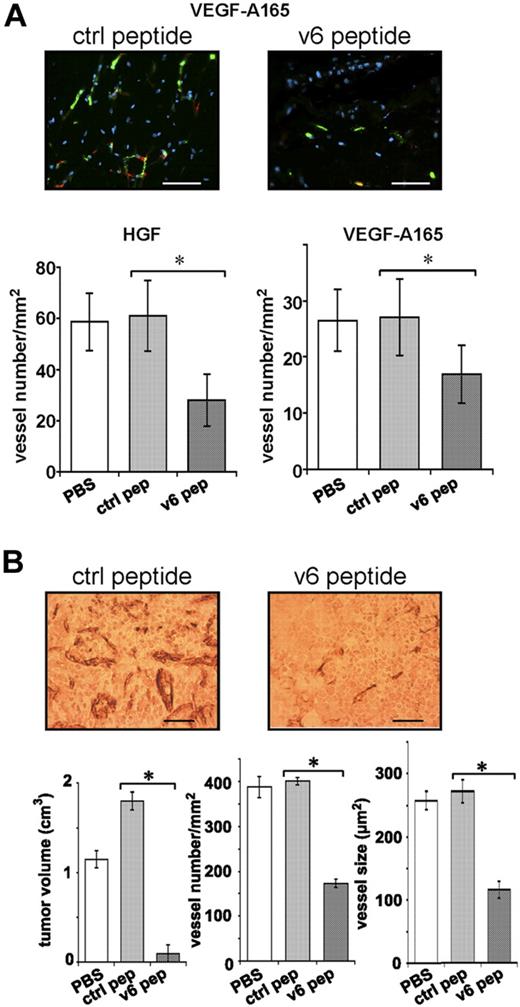
A human vasculature can be engineered from human EC spheroids embedded together with growth factors in Matrigel/fibrin on grafting into the flank of SCID mice.24 We have used this assay to test whether CD44v6 is involved in the development of this vasculature. HUVEC spheroids embedded in Matrigel/fibrin containing either HGF or VEGF-A165, together with the CD44v6 peptide (or a control peptide), were subcutaneously injected into the flank of SCID mice. The formation of the human vascular network was determined 3 weeks later by staining of the Matrigel/fibrin plugs with human CD34 antibodies. Both HGF and VEGF induced vessel formation (Figure 7A), an effect that was not detectable in the absence of either growth factor (not shown). A CD44v6 peptide significantly reduced the angiogenic response to more than 50% (Figure 7A), whereas a control peptide had no effect. Thus, also in this assay, the angiogenic response is dependent on CD44v6. Furthermore, these results confirm that HGF can act as an angiogenic factor.8-12
The formation of a vasculature in vivo requires CD44v6. (A) A spheroid-based in vivo angiogenic assay was performed as described in “In vivo angiogenesis assay.” Growth factor and peptide treatments are indicated. Two representative pictures of blood vessels in plugs are shown. Bars represent 50 μm. Staining of blood vessels was performed with CD34 antibody (green); red indicates smooth muscle actin staining (including pericytes; Sigma-Aldrich). Nuclei were stained with Hoechst dye 33258 (blue; Sigma-Aldrich). A minimum of 3 different plugs per condition were analyzed and quantified by immunologic staining (“Immunohistologic analysis”). (B) L3.6pl human pancreatic cancer cells were injected orthotopically into male nude mice as described.18 Three groups of 5 mice each were injected 7 days later with PBS or control peptide (20 μg) or CD44 v6 peptide (20 μg). Injection was repeated 3 times per week. Animals were killed 21 days after the beginning of the treatment. Tissues were stained with the endothelial marker CD31 (“Immunohistologic analysis”). A representative staining is shown. Bars represent 50 μm. Tumor volume, vessel number, and average vessel size were determined and evaluated in the graphs. All animals were handled according to German regulations for animal experimentation. The animal experiments were approved by the Regierungspäsidium Karlsruhe (35-9185.81/G-83/04). All mice were obtained from Harlan.
The formation of a vasculature in vivo requires CD44v6. (A) A spheroid-based in vivo angiogenic assay was performed as described in “In vivo angiogenesis assay.” Growth factor and peptide treatments are indicated. Two representative pictures of blood vessels in plugs are shown. Bars represent 50 μm. Staining of blood vessels was performed with CD34 antibody (green); red indicates smooth muscle actin staining (including pericytes; Sigma-Aldrich). Nuclei were stained with Hoechst dye 33258 (blue; Sigma-Aldrich). A minimum of 3 different plugs per condition were analyzed and quantified by immunologic staining (“Immunohistologic analysis”). (B) L3.6pl human pancreatic cancer cells were injected orthotopically into male nude mice as described.18 Three groups of 5 mice each were injected 7 days later with PBS or control peptide (20 μg) or CD44 v6 peptide (20 μg). Injection was repeated 3 times per week. Animals were killed 21 days after the beginning of the treatment. Tissues were stained with the endothelial marker CD31 (“Immunohistologic analysis”). A representative staining is shown. Bars represent 50 μm. Tumor volume, vessel number, and average vessel size were determined and evaluated in the graphs. All animals were handled according to German regulations for animal experimentation. The animal experiments were approved by the Regierungspäsidium Karlsruhe (35-9185.81/G-83/04). All mice were obtained from Harlan.
Vascularization of a pancreatic tumor requires CD44v6
To prove whether tumor-induced angiogenesis can be repressed by the CD44v6 peptide, we made use of human pancreatic carcinoma cells (L3.6pl).18 These cells have already been used to study angiogenesis on orthotopic injection in mice.30,31 L3.6pl cells were injected into the tail of the pancreas of SCID mice. One week later, a CD44v6 peptide (or a control peptide) was administered intraperitoneally 3 times per week for 3 weeks.
In the previous experiments, we have used a CD44v6 human peptide and a human antibody to target human ECs. In the L3.6pl tumor model, however, we used a mouse-specific peptide. CD44v6 peptides are species specific and do not cross-react (A.M., V.O.-R., unpublished data, May 2008).15 In particular, the mouse CD44v6 peptide does not interfere with CD44 functions on tumors cells (human). Thus, it targeted only angiogenesis of host ECs. We observed a drastic inhibitory effect of the CD44v6 peptide on the growth of the human pancreatic tumors established from L3.6pl cells: the tumor size was drastically reduced (Figure 7B). Furthermore, the vessel density and the vessel size were decreased by more than 60% on treatment with the CD44v6 peptide (Figure 7B). These data suggest that the growth of this pancreatic tumor is particularly dependent on the establishment of a blood vasculature, although we cannot exclude additional effects of the peptide on non-ECs.
In conclusion, we have shown that the CD44v6 peptide and antibody not only block the activation and signaling of VEGFR-2 on several ECs and thereby interfere with tubular outgrowth and EC migration in a variety of test systems but that they also inhibit tumor-induced blood vessel formation in vivo.
Discussion
CD44 proteins have already been shown to be relevant for angiogenesis: angiogenesis is impaired in CD44 null mice.17 bFGF and VEGF, 2 important angiogenic factors, up-regulate CD44 on ECs in vivo, and targeting of CD44 by specific antibodies leads to EC killing.32 CD44-specific antibodies repress EC proliferation and capillary formation in fibrin matrix.33 The proliferation of ECs and their adhesion to hyaluronan, a component of the ECM, are dependent on CD44.34 CD44 together with bFGF is involved in tubule formation of ECs in collagen gels.35 Finally, CD44v3 was detected in ECs and v3-specific antibodies blocked chemotaxis of these cells.36 Interestingly, low molecular weight hyaluronan, a degradation product of matrix hyaluronan released on tissue injury and inflammation, stimulates EC proliferation by binding to CD44. This leads to activation of the MAP kinase pathway and subsequent induction of early response genes.37,38 Our findings here explain mechanistically some of these observations.
Indeed, here we show that the activation of c-Met and VEGFR-2 by their respective ligands in ECs is strictly dependent on a CD44 isoform containing the variant exon v6. In HUVECs, a v6 peptide and a CD44v6ECD abrogate both c-Met and VEGFR-2 activation and subsequent induction of EC migration, spheroid sprouting, and tubule formation. Coimmunoprecipitation studies show that VEGFR-2 and CD44v6 form a complex and seem to be constitutively associated with each other. The dependency of VEGFR-2 toward CD44v6 is not only true in HUVECs but also in HAOECs and HCMECs and can be simulated in other cells (HEK293 cells and BSp73ASs6 cells) transfected to express VEGFR-2.
VEGFR-2 is a key receptor for the development of the blood vasculature. Vegfr-2 knockouts are lethal because of a deficiency in blood vessel formation.39 In contrast, CD44-deficient mice develop normally and have only minor immunologic defects.40 How do these observations fit to the finding described here that the activation of VEGFR-2 is strictly dependent on CD44v6? This situation resembles the one found for the c-Met receptor where the embryonic lethality of c-Met deficient mice is also in striking contrast to the phenotype of CD44-deficient mice.41 The most plausible explanation for these discrepancies is that the function of CD44 and, in particular, the coreceptor function of CD44v6, is substituted by another protein in CD44 null mice. We have recently published genetic evidence for such a substitution.42 Meanwhile, we have also identified a substituting molecule for CD44v6 in CD44 null mice (V.O., V.O.-R., unpublished results, January 2007) confirming further our hypothesis.
The fact that the CD44v6 peptide blocks such different RTKs as c-Met and VEGFR-2 is puzzling. That it has no unspecific effect on receptor activation and angiogenesis is demonstrated by the fact that PDGFR activation and PDGF-dependent tubule formation by ECs are not blocked by the peptide. An explanation for its effect on 2 different receptors might be the finding that the CD44v6 peptide addresses CD44v6 itself by changing its conformation (A.M., V.O.-R., unpublished results, January 2008) and thereby does not directly interfere with an interaction between CD44v6 and the RTKs or their ligands.
In addition to its role in the activation of VEGFR-2, CD44v6 is also necessary for intracellular signal transduction. For the activation of the MAPK pathway, the CD44v6 cytoplasmic domain appears to recruit ERM proteins and the cytoskeleton to allow Ras activation by its GEF SOS similarly to what has been shown for c-Met in epithelial cells.16 This is particularly interesting because the MAPK pathway plays a crucial role in angiogenesis for proliferation,43 survival,44 and migration45 of ECs. Furthermore, Mek1 knockout mice are embryonic lethal and die from a placental defect caused by impaired angiogenesis.46 Recently, the MAPK pathway has been shown to induce EC survival and sprouting by inhibiting Rho.47
VEGF has been described as a heparin-binding growth factor and has been shown to bind to CD44 exon v3-containing isoforms that carry HS.27 Here we show that activation of VEGFR-2 can be induced also by the non–heparin-binding VEGF-A121 and that this activation is also dependent on the CD44 exon v6. Furthermore, although ECs express CD44 exon v3 and exon v6, these exons seem not to be present on the same protein as deduced from RT-PCR analysis. Finally, activation of VEGFR-2 was observed in BSp73ASs6 cells that express only CD44v6 and CD44s but not the CD44v3 HS form. These results indicate that activation of VEGFR-2 per se may not rely on HS. It seems, however, that binding to HS is required for a full-blown angiogenic response because VEGF-A121 is a less potent EC mitogen48 and has a 10- to 100-fold lower biologic activity than VEGF-A165.49 Neuropilin 1, an HS-modified protein that can bind VEGF-A165 but not VEGF-A121,50 seems a good candidate to provide HS moieties.51 Hence, cooperation between VEGFR-2, VEGF, neuropilin, and CD44v6 is probably involved in a full-blown angiogenic response.
The coreceptor function of CD44v6 for VEGFR-2 is highly relevant in tumor angiogenesis, as shown by the effect of the CD44v6 peptide and the CD44v6 antibody on a human pancreatic tumor. The tumor size as well as microvessel density and average vessel size are drastically decreased after treatment with the CD44v6 peptide.
There is ample evidence that the CD44 family of proteins plays an important role in tumorigenesis. An impressive number of publications report the relevance of CD44s and CD44 variants as prognostic markers for human cancers.52 Moreover, in a rat pancreatic carcinoma cell system, transfection of CD44v6 containing isoforms converted nonmetastatic cells into metastasizing ones. Conversely, CD44v6-specific antibodies inhibited metastasis.14 Our findings here, that CD44 isoforms are relevant for angiogenesis, add a new dimension to the role of CD44 in tumorigenesis.
The CD44v6 peptide might be used as an angiogenic inhibitor. Various approaches were attempted to interfere with angiogenesis. One of the most famous drug is bevacizumab, also known as avastin, a humanized, function-blocking monoclonal antibody that selectively neutralizes VEGF-A.5 The advantage of using a CD44v6 peptide for cancer therapy could be several-fold. First, according to their small size (14mer or even 5mer), they can be readily produced, they are unlikely to induce an immune response, and they can be easily delivered to their target sites. In addition, they can block several RTKs, are efficient against angiogenesis and metastasis, and might be effective in several types of tumors.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank George Vande Woude (Van Andel Institute) for providing HGF and C. Bruns (University of Munich) for providing us with the L3.6pl cells.
This work was supported by the Deutsche Forschungsgemeinschaft (Priority Research Program SPP1190; The tumor-vessel interface) and the Mildred Scheel Foundation. H.G.A. is supported by an endowed chair from the Avantis Foundation.
Authorship
Contribution: M.T. designed and performed most of the research; A.M., I.A., A.M.L., and V.O. designed and performed part of the research; K.B.-H. provided vital new reagents and critically reviewed the manuscript; G.C. designed part of the research and critically reviewed the manuscript; M.H. and H.G.A. designed part of the research; H.P. designed part of the research, analyzed the data, and critically reviewed the manuscript; and V.O.-R. designed the research, analyzed the data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Véronique Orian-Rousseau, Forschungszentrum Karlsruhe, Institute for Toxicology and Genetics, Postfach 3640, 76021 Karlsruhe, Germany; e-mail: veronique.orian-rousseau@itg.fzk.de.