Abstract
Abstract 715
We previously reported interim results (Blood-ASH Annual Meeting Abstracts-2007 110: abstract 2324) of a prospective study evaluating dose-dense and dose-intense variants of ABVD regimen. Seventy pts with a newly diagnosis of cHL were enrolled from 06/04 to 03/08. Pts had intermediate-(INT) or advanced-stage (ADV) according to GHLSG criteria (Tab.1). INT (n=24) were treated with dd ABVD, and ADV (n=46) with dd-di ABVD. Briefly, the strategy concepts of treatment were: 6 cycles of chemotherapy; the inter-cycle period shortened from 28 to 21 days; drugs delivered at day 1 and 11 of each cycle; In the dd-di-ABVD, adriamycin was escalated from 50 to 70 mg/m2 in cycles 1-4; primary G-CSF was given; the therapy was driven by interim-FDG-PET; radiotherapy was planned with very stringent criteria.
Presentation Features
| . | Intensified ABVD . | Baseline ABVD . | ||
|---|---|---|---|---|
| N . | % . | N . | % . | |
| stage II (*) | 24 | 34 | 25 | 36 |
| stage II (**) | 11 | 16 | 9 | 13 |
| stage III | 9 | 13 | 9 | 13 |
| stage IV | 26 | 37 | 27 | 38 |
| GHLSG stage | ||||
| ADV-stage | 46 | 34 | 45 | 36 |
| INT-stage | 24 | 66 | 25 | 64 |
| male | 23 | 33 | 24 | 34 |
| age>45-yr | 10 | 14 | 11 | 16 |
| Bulky disease | 33 | 47 | 34 | 49 |
| B symptoms | 45 | 64 | 48 | 69 |
| E-disease | 27 | 39 | 25 | 36 |
| ESR >50 mm | 30 | 43 | 32 | 46 |
| LDH ratio>1 | 25 | 36 | 24 | 34 |
| IPS ≥ 3 | 23 | 33 | 25 | 36 |
| N sites ≥ 4 | 49 | 70 | 50 | 72 |
| . | Intensified ABVD . | Baseline ABVD . | ||
|---|---|---|---|---|
| N . | % . | N . | % . | |
| stage II (*) | 24 | 34 | 25 | 36 |
| stage II (**) | 11 | 16 | 9 | 13 |
| stage III | 9 | 13 | 9 | 13 |
| stage IV | 26 | 37 | 27 | 38 |
| GHLSG stage | ||||
| ADV-stage | 46 | 34 | 45 | 36 |
| INT-stage | 24 | 66 | 25 | 64 |
| male | 23 | 33 | 24 | 34 |
| age>45-yr | 10 | 14 | 11 | 16 |
| Bulky disease | 33 | 47 | 34 | 49 |
| B symptoms | 45 | 64 | 48 | 69 |
| E-disease | 27 | 39 | 25 | 36 |
| ESR >50 mm | 30 | 43 | 32 | 46 |
| LDH ratio>1 | 25 | 36 | 24 | 34 |
| IPS ≥ 3 | 23 | 33 | 25 | 36 |
| N sites ≥ 4 | 49 | 70 | 50 | 72 |
stage IIA with E and/or bulky disease and/or nodal sites>3 and/or B symptoms
stage II B with E and/or bulky disease
On a total of 838 courses of chemo we had 18 events which needed red cell transfusion; 5 of severe thrombocytopenia completely reversed within one week; 25 of severe neutropenia (<500 mmc) at the recycle. In 9 events pts were hospitalized to treat infections. Reversible palmar-plantar erythrodysesthesia was seen in 27 pts.The onset was between the 2nd and 4th cycle and disappeared within one month from the end of therapy (Tx). Three reversible events mimicking acute abdominal emergency needed a brief hospitalisation for support therapy. A suspect drug's alveolitis was seen in 3 events. This condition appeared between the 5th/6th cycles and was successfully treated with support. In all cases pts continued the Tx with a minimum delay and a moderate dose reduction of bleomycin. One case of sporadic reversible epileptogenic syndrome was seen in a 16-yr old girl. Early(1-yr) and late(4-yr) cardiac toxicity was studied in 70 and 25 pts, respectively: there was no evidence of relevant cardiac dysfunction.
Median duration of chemotherapy (planned 18-wk) was 19.7-wk (range 17.6-21.7). Planned and delivered RDIs of drugs were significantly higher as compared with the most used regimens (Tab.2).
Schedules and dose-density and intensity of Adriamycin
| . | ADR mg/m2 . | Recycle weeks . | Dose-intensity mg/m2/wk . | RDI* . | Cum. dose mg/m2 . |
|---|---|---|---|---|---|
| Hybrid MOPP-ABV | 35 | 4 | 8.75 | 0.70 | 280 8 cy; 4 doses |
| Baseline BEACOPP | 25 | 3 | 8.33 | 0.66 | 200 8 cy; 8 doses |
| Escalated BEACOPP | 35 | 3 | 11.66 | 0.93 | 280 8 cy; 8 doses |
| BEACOPP 14 | 25 | 2 | 12.5 | 1 | 200 8 cy; 8 doses |
| Stanford V | 25 | 2 | 12.5 | 1 | 150 12 wks; 6 doses |
| Baseline ABVD | 25 | 2 | 12.5 | 1 | 400 8 cy; 16 doses |
| dd ABVD | 25 | 1½ | 16.66 | 1.33 | 300 6 cy; 12 doses |
| Delivered: mean (range) | 1.21 (1.14-1.25) | ||||
| dd-di ABVD | 35 | 1½ | 21.11 | 1.69 | 380 6 cy; 12 doses |
| Delivered: mean (range) | 1.54 (1.39-1.74) |
| . | ADR mg/m2 . | Recycle weeks . | Dose-intensity mg/m2/wk . | RDI* . | Cum. dose mg/m2 . |
|---|---|---|---|---|---|
| Hybrid MOPP-ABV | 35 | 4 | 8.75 | 0.70 | 280 8 cy; 4 doses |
| Baseline BEACOPP | 25 | 3 | 8.33 | 0.66 | 200 8 cy; 8 doses |
| Escalated BEACOPP | 35 | 3 | 11.66 | 0.93 | 280 8 cy; 8 doses |
| BEACOPP 14 | 25 | 2 | 12.5 | 1 | 200 8 cy; 8 doses |
| Stanford V | 25 | 2 | 12.5 | 1 | 150 12 wks; 6 doses |
| Baseline ABVD | 25 | 2 | 12.5 | 1 | 400 8 cy; 16 doses |
| dd ABVD | 25 | 1½ | 16.66 | 1.33 | 300 6 cy; 12 doses |
| Delivered: mean (range) | 1.21 (1.14-1.25) | ||||
| dd-di ABVD | 35 | 1½ | 21.11 | 1.69 | 380 6 cy; 12 doses |
| Delivered: mean (range) | 1.54 (1.39-1.74) |
RELATIVE DOSE INTENSITY Baseline ABVD=1
Early-CR (PETneg > 2 cycles) was obtained in 65/70 pts (95%). No statistical differences was noted between INT and ADV subsets. At the end of 6thcycle 69/70 pts (98,6%) were in CR. Three out of 69 complete responders (4.3%) had a biopsy-proven relapse: a 33-yr old man ( IVEA), a 27-yr old girl (IIB) and 29-yr old girl ( IVXEB). Relapsed occurred at 3, 10 and 14 months from the end of Tx, respectively.
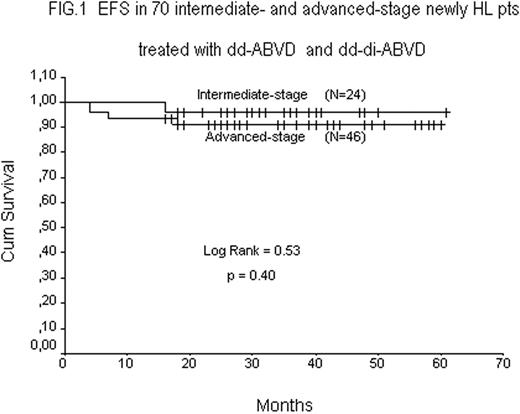
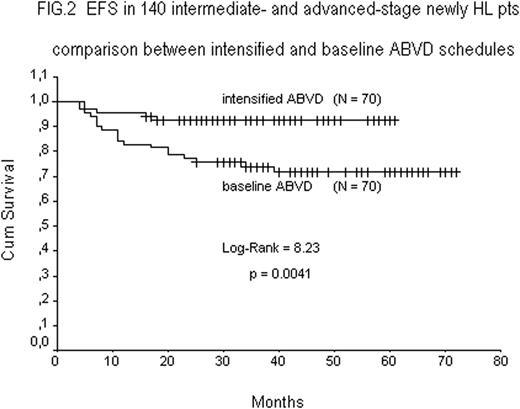
Data with a minimum follow up of 12-mo from the end of Tx were available in all 70 pts. Fig.1 shows the EFS rates of 24 INT-stage (95.8%), and 46 ADV-stage (91.3%) pts, respectively. A comparative analysis (Fig.2) between this series of 70 pts treated with dd ABVD or dd-di ABVD and the last 70 historical INT-stage (n=25) and ADV-stage (N=45) pts treated with baseline ABVD shows a statistically significant increment in EFS rate in pts receiving intensified ABVDs (93.0% vs 73.2% p=0.0041).
The final results of this study shows that the activity of intensified ABVD is significantly higher than baseline ABVD in terms of response (CR) and survival (EFS) rates, still maintaining a low-toxicity profile. Based on these results a randomised comparison of intensified versus baseline ABVD seems justified.
Response and Survival
| . | Intensified . | standard . | stat . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| . | dd ABVD . | dd-di ABVD . | total . | baseline ABVD . | |||||
| End-point . | N . | % . | N . | % . | N . | % . | N . | % . | p . |
| Early-CR | 23/24 | 96 | 42/46 | 91 | 65/70 | 93 | - | - | |
| CR | 24/24 | 100 | 45/46 | 98 | 69/70 | 99 | 62/70 | 89 | ns |
| 5-yr EFS | 23/24 | 96 | 42/46 | 91 | 65/70 | 93 | 51/70 | 73 | 0.0041 |
| . | Intensified . | standard . | stat . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| . | dd ABVD . | dd-di ABVD . | total . | baseline ABVD . | |||||
| End-point . | N . | % . | N . | % . | N . | % . | N . | % . | p . |
| Early-CR | 23/24 | 96 | 42/46 | 91 | 65/70 | 93 | - | - | |
| CR | 24/24 | 100 | 45/46 | 98 | 69/70 | 99 | 62/70 | 89 | ns |
| 5-yr EFS | 23/24 | 96 | 42/46 | 91 | 65/70 | 93 | 51/70 | 73 | 0.0041 |
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal