Abstract
Abstract 557
Venous thromboembolism (VTE), which includes deep vein thrombosis (DVT) and pulmonary embolism (PE), is an important clinical event that needs to be accurately reported. The Agency for Healthcare Research and Quality (AHRQ) has categorized VTE as a reportable patient safety indicator (PSI) and has distributed a software package for recording events such as VTE based upon diagnosis codes. At our institution, there was concern that VTEs identified by this software method may be over estimating the actual number.
To verify the cases of VTE as identified by AHRQ methodology through a review of the medical record for each event reported.
Through a review of the electronic medical record (EMR), the events recorded as a VTE by PSI software over a 7 month period from November 2008 to May 2009 were further characterized into categories of acute, remote or age indeterminate DVTs and were distinguished from events that were either isolated superficial vein thrombosis (SVT) or that were not VTE at all. Discharge summaries, hospital notes, operative reports and imaging reports were used to verify identified cases of VTE. VTE occurrences were grouped by the month of discharge of the patient. The PSI software utilized diagnosis codes that have been abstracted for a patient's hospitalization based upon physician documentation.
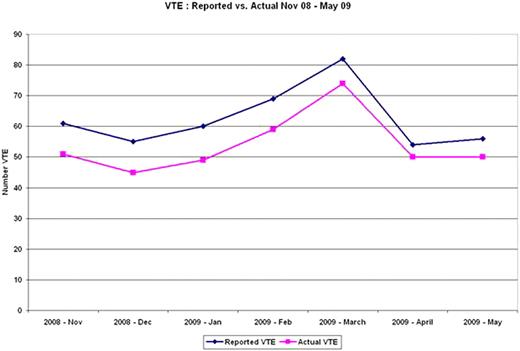
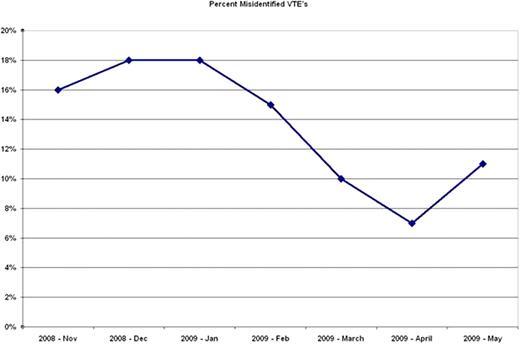
437 events were recorded as VTE over a 7 month period. 59 cases (13.5%) were incorrectly coded as VTE. 35 cases (8.0%) were superficial vein thrombosis mistakenly classified as DVT. 12 cases (2.7%) had remote or age indeterminate DVTs. 12 cases (2.7%) cases had no VTE and were considered further abstraction errors. After interim reporting of the first 4 months of VTE data, vascular laboratory study reports were amended so that superficial vein thrombosis would no longer be identified by this terminology and would subsequently be classified as superficial vein thrombophlebitis. The last three months of data collection had 9 cases (4.7%) incorrectly identified as DVTs that were actually SVTs versus 26 cases (10.6%) prior to implementation of the nomenclature change.
Hospitals must be aware of reporting practices in their own institution as national protocols may not completely depict individual hospital occurrences. Careful attention to the manner in which the medical record reflects events can make substantial difference in accounting of VTE occurrence. Because of the lack of specificity of current diagnosis coding guidelines, an appropriate medical term was not sufficient for medical record abstractors to categorize an event correctly in this study. As hospitals become monitored for quality of care with these methodologies, the accuracy of reporting becomes paramount.
Summary of VTE Data (Nov 08–May 09)
| . | Nov . | Dec . | Jan . | Feb . | Mar . | Apr . | May . |
|---|---|---|---|---|---|---|---|
| VTE Cases Reported | 61 | 55 | 60 | 69 | 82 | 54 | 56 |
| Age Indeterminate/Remote DVTs | 1 | 1 | 1 | 3 | 2 | 2 | 2 |
| SVTs | 6 | 6 | 8 | 6 | 5 | 0 | 4 |
| Non-VTE | 3 | 3 | 2 | 1 | 1 | 2 | 0 |
| Total Mistaken Reporting of VTE | 10 (16.4%) | 10 (18.2%) | 11 (18.3%) | 10 (14.5%) | 8 (9.8%) | 4 (7.4%) | 6 (10.7%) |
| . | Nov . | Dec . | Jan . | Feb . | Mar . | Apr . | May . |
|---|---|---|---|---|---|---|---|
| VTE Cases Reported | 61 | 55 | 60 | 69 | 82 | 54 | 56 |
| Age Indeterminate/Remote DVTs | 1 | 1 | 1 | 3 | 2 | 2 | 2 |
| SVTs | 6 | 6 | 8 | 6 | 5 | 0 | 4 |
| Non-VTE | 3 | 3 | 2 | 1 | 1 | 2 | 0 |
| Total Mistaken Reporting of VTE | 10 (16.4%) | 10 (18.2%) | 11 (18.3%) | 10 (14.5%) | 8 (9.8%) | 4 (7.4%) | 6 (10.7%) |
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal