Abstract
Abstract 543
Recombinant factor VIIa (rFVIIa) is an analogue of the naturally occurring procoagulant and has a narrow indication for usage including patients with hemophilia and an inhibitor or factor VII deficiency. In adults, off-label indications account for most rFVIIa usage despite inadequate studies demonstrating safety, dosing and efficacy. Significant concerns exist regarding the thrombotic potential of rFVIIa and the associated morbidity and mortality. The objective of this study was to describe the use of rFVIIa in hospitalized pediatric patients and to assess thrombotic outcomes.
This retrospective multicenter cohort study used data from 40 free-standing children's hospitals that contribute data to the Pediatric Health Information System administrative database. Children less 18 years of age who received rFVIIa, as determined through pharmacy billing data, between 2000-2007 were included. A label admission was identified when there was an International Classification of Diseases (ICD-9) diagnostic code for hemophilia or Factor VII deficiency; admissions without these codes were classified as off-label. ICD-9 discharge codes were used to identify admissions during which an arterial or venous thromboembolism was diagnosed. Continuous data were analyzed with the student t-test and categorical data with the chi squared test. Poisson regression was used to analyze the rate of rFVIIa use over time.
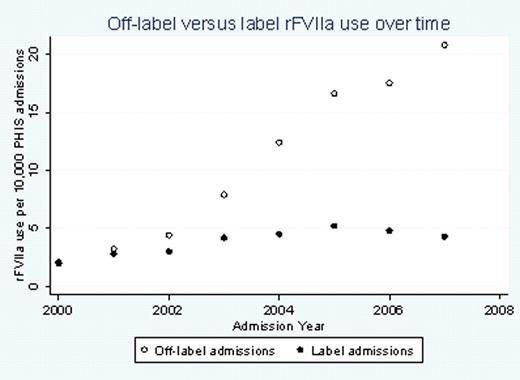
There were a total of 4,942 admissions in which rFVIIa was used, representing 3,764 individual subjects. rFVIIa was used off-label in 74% (3655) of the admissions. There was a 10-fold increase in the annual rate of off-label admissions from 2000 to 2007 (from 2 to 20.8 per 10,000 hospital admissions, p<.0001) (figure 1). Forty percent (1455/3655) of the off-label rFVIIa use occurred in children less than one year of age. The most frequent attending physician specialties for the off-label admissions included hematology/oncology 16.8%, critical care 16.1%, cardiology 10.3%, cardiovascular/thoracic surgery 10.2%, and neonatology 7.4%. In most admissions (88%), the ICD-9 discharge codes included one or more complex chronic conditions (CCC); the most common CCCs were cardiovascular (30%), hemophilia (24%), and malignancy (14%). The mean length of stay was 35.5 +/- 47 days for the off-label admissions. The off-label admissions had a lower mean of the total days of rFVIIa use, 2.3 +/- 4.7, compared to label admissions with 5.1 +/- 5.5 days (p<.001). The mortality rate in the off-label group was 34% (1258/3655) versus 1.6% (21/1287) in the label group (p<.001). Thrombotic events, the majority of which were venous, occurred in 7.7% (283/3655) of the off-label admissions and in 2.3% (30/1287) of the label admissions.
The off-label use of rFVIIa in hospitalized children is increasing rapidly despite the absence of adequate clinical trials demonstrating safety and efficacy. The mortality rate in patients who receive off-label rFVIIa is extremely high, and the proportion of patients who develop a thrombosis is not trivial. Further prospective studies are urgently needed to address the safety and efficacy of this drug.
Off Label Use: This paper will report on the off-label use of rFVIIa in pediatric patients.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal