Abstract
Abstract 5107
Many thalassemia major (TM) patients have concomitant hepatitis B and/or C infection and data suggest that iron overload in these patients increases the risk of liver fibrosis progression. Deferasirox (Exjade®), which has demonstrated efficacy and safety in reducing iron overload in a variety of transfusion dependent anemias, has been uncommonly associated with elevations of transaminases suggestive of hepatitis (0.3%). However, whether patients' viral hepatitis status affects the overall and liver safety of deferasirox remains unclear. The prospective, multicenter EPIC study of deferasirox enrolled some patients with a history of hepatitis B or C. This sub-analysis of EPIC assesses the overall and liver safety of deferasirox over 1 year of treatment in TM patients with and without a history of hepatitis B or C.
Patients aged ≥2 years with transfusion-dependent TM and serum ferritin (SF) levels of ≥1000 ng/mL, or <1000 ng/mL with a history of multiple transfusions (>20 transfusions or >100 mL/kg of red blood cells), or R2 MRI-confirmed liver iron concentration of >2 mg Fe/g dry weight were enrolled. Deferasirox starting dose was 10–30 mg/kg/day depending on the frequency of blood transfusions. Protocol-specified dose adjustments of 5–10 mg/kg/day (range 0–40) were performed every 3 months based on SF trends and safety markers. Safety was evaluated through continuous monitoring and recording of adverse events (AEs), as well as monthly blood chemistry assessments including alanine aminotransferase (ALT) and aspartate aminotransferase (AST).
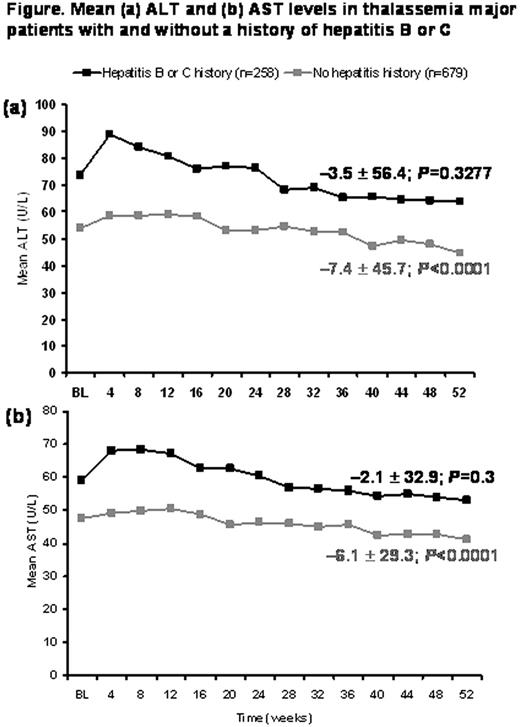
Of 937 enrolled patients with TM, 258 (27.5%) had a history of hepatitis B or C and 679 (72.5%) did not. Compared with patients with no viral hepatitis, patients with a history of hepatitis B or C were older (mean age 26.5 ± 9.9 vs 15.3 ± 9.5 years), more commonly splenectomized (50.4 vs 29.9%), received more transfusions (195 vs 188 mL/kg) and were chelated for longer prior to study entry (15.9 ± 10.5 vs 8.7 ± 7.4 years). In total, 212 (82.2%) and 636 (93.7%) of patients with and without viral hepatitis, respectively completed the study. The most common drug-related AEs were gastrointestinal disorders, such as diarrhea (14.7% vs 6.2%, respectively; P<0.0001), abdominal pain (7.4% vs 4.7%, respectively; P=0.110) and nausea (5.0% vs 3.5%, respectively; P=0.291), as well as skin rash (10.5% vs 13.5%, respectively; P=0.205). Three patients (1.2%) with hepatitis had two consecutive increases in ALT 10 x the upper limit of normal (ULN); all three patients had elevated baseline levels (mean 95.6 U/L). Two patients (0.3%) without hepatitis had similar increases, one of whom had elevated baseline levels (225 U/L). Mean ALT and AST levels decreased during deferasirox treatment in both groups; decreases were significant in patients without hepatitis (Figure). 12 (4.7%) and 25 (3.7%) patients with and without a history of viral hepatitis, respectively had serum creatinine >33% above baseline and ULN on two consecutive visits
Overall median SF decreased from baseline (3157 ng/mL) by 129 ng/mL after 1 year (P=0.0007) at a mean actual dose of 24.2 ± 5.6 mg/kg/day and mean transfusional iron intake of 0.43 ± 0.2 mg/kg/day. Reduction in SF was similar in patients with or without a history of viral hepatitis (–113 and –148 ng/mL, respectively).
This sub-analysis of EPIC demonstrates that once-daily deferasirox is well tolerated in patients with TM irrespective of viral hepatitis history with no significant difference in the incidence of abdominal pain, nausea and skin rash. Increases in ALT >10 x ULN were very low and occurred at a comparable frequency in patients with and without viral hepatitis. Mean liver transaminases, although higher at baseline in patients with a history of hepatitis B or C compared to those without, decreased in both groups, suggesting an overall improvement in liver function with deferasirox treatment. Reduction in SF was similar in both patient groups. Further confirmatory data are required to evaluate the effect of hepatitis viral load on the response to deferasirox therapy.
Cappellini:Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Genzyme: Membership on an entity's Board of Directors or advisory committees. Kattamis:Novartis: Consultancy, Honoraria, Speakers Bureau. Chan:Novartis: Honoraria, Research Funding. Lin:Taiwan Pediatric Onclogy Group (TPOG): Consultancy; Novartis: Honoraria, Speakers Bureau. Sutcharitchan:Novartis: Honoraria, Research Funding; Novo Nordisk: Honoraria. Taher:Novartis: Honoraria, Research Funding. Habr:Novartis Pharmaceuticals: Employment. Domokos:Novartis Pharma AG: Employment. Roubert:Novartis Pharma AG: Employment. Porter:Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Vifor International: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal