Abstract
Abstract 506
In a majority of chronic myeloid leukemia patients treatment with imatinib (IM) induces durable hematologic, cytogenetic, and molecular responses, which in turn results in a dramatic improvement of progression-free survival. A new challenge for further optimizing the management of CML is to determine if treatment with TKIs such as IM could eventually cure the patients, particularly those with sustained undetectable disease, by reducing or eliminating the leukemic self-renewing cell (LSC) population thought to drive the disease. Given the challenges in directly assessing LSC burden in patients, we have applied mathematical modeling to molecular response data from patients on IM therapy to infer whether LSC levels can be reduced during IM treatment.
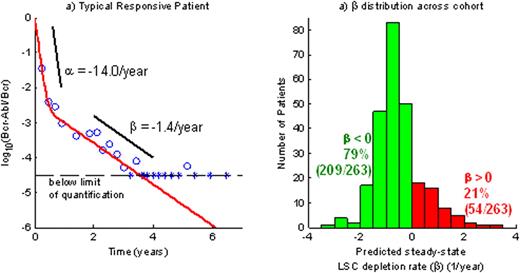
To conduct our study we have utilized 281 patients on the IM arm of the International Randomized Study of Interferon Versus STI571 (IRIS) trial (Drucker et al., NEJM, 2006; 355, 2408). The 281 patients maintained a 90% dose intensity over the course of treatment (up to 7 years) and had a sufficient number of PCR samples above the quantitation limit to support parameter estimation. We have modeled the Bcr-Abl/Bcr transcript ratio time course R(t) as a biexponential (R(t) = Aeαt + Beβt ) and estimated the model parameters for each patient using maximum likelihood methods. The parameter α < 0 describes the rapid initial decline in log10(R) upon treatment start while β describes the shallower slope of the subsequent log10(R) kinetics (figure 1a).
Figure 1a (left): illustration of alpha and beta slopes in a typical responsive patient
Figure 1b (right): distribution of beta slopes in the study population
Figure 1a (left): illustration of alpha and beta slopes in a typical responsive patient
Figure 1b (right): distribution of beta slopes in the study population
nearly every patient response trajectory (93%-263/281) was well-described by the bi-exponential model. The key parameter β, corresponding to the steady-state per-year reduction in log10 transcript levels, ranged from a minimum = -9.2, lower quartile = -1.1, median = -0.6, upper quartile = -0.2, maximum = 10.5 (see figure 1b). Approximately 21% (54/263) of patients had β>0, suggestive of molecular relapse, while 79% (209/263) have β<0, corresponding to continued transcript decline.
The durable reduction in Bcr-Abl transcripts in the majority of patients has two possible interpretations: either LSCs are depleted at a rate β, or LSCs are not depleted but leukemic progenitor cells in the bone marrow have a median half-life of over one year in many patients. However, leukemic progenitor dynamics appear to occur at a time scale of months (Abe et al, Int J Hematol. 2008; 88, 471); thus a durable reduction in transcript level must correspond to a depletion of LSCs. Our hypothesis that IM induces LSC depletion is also consistent with the observation that 50% of patients maintain undetectable Bcr-Abl transcript levels upon treatment discontinuation (Guastafierro et al, Leukemia Res. 2009; 33, 1079).
Mathematical modeling which combines pathophysiologic principles of CML with 7-year molecular response data in individual patients predicts that most patients (∼79%) experience reduction in LSC burden while on sustained IM treatment. In the future, this modeling approach can be used to analyze data from patients who stop IM after achieving CMR in the context of carefully conducted clinical trials, with the goal of assessing (a) the potential impact of IM interruption on LSC levels, particularly in those patients who relapse upon stopping IM and then are re-induced, and (b) whether the probability of achieving cure depends on duration of CMR, the steepness of response prior to achieving CMR (β), and/or additional factors.
Stein:Novartis: Employment. Kalebic:Novartis: Employment. Bottino:Novartis: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal