Abstract
Abstract 4938
Bortezomib (Velcade®) is effective and well tolerated in patients with multiple myeloma (MM), including those with adverse prognostic factors such as advanced age, more prior lines of therapy, and advanced-stage disease. The international, non-interventional, Electronic Velcade Observational Study (eVOBS) is an ongoing observational study that aims to assess the clinical and health economic outcomes in MM patients treated with bortezomib in the clinical-practice setting. In this study, we characterized patients achieving complete response (CR) with bortezomib in the relapsed/refractory setting, and assessed the effects of adverse prognostic factors on the rate of CR.
Adult patients from Belgium, France, Greece, Russia, Spain, Sweden, and Turkey scheduled to receive bortezomib for relapsed MM were eligible for inclusion in eVOBS. All bortezomib doses and concomitant treatments (except investigational therapies) were permitted; dose adjustments and cycle delays were documented. Patients are being followed for up to 3 years to document long-term outcomes; data are collected at baseline and at the end of every bortezomib cycle. Due to the non-interventional nature of the study, no predefined response criteria were mandated; response criteria were defined by the investigator and may have included European Group for Blood and Marrow Transplantation (EBMT), Southwest Oncology Group (SWOG), or M-protein criteria. Patients who had at least 12 weeks data or who had died by the time of data cut-off (June 30, 2009) were included in this exploratory analysis; patients were assessed after completion of four and six cycles.
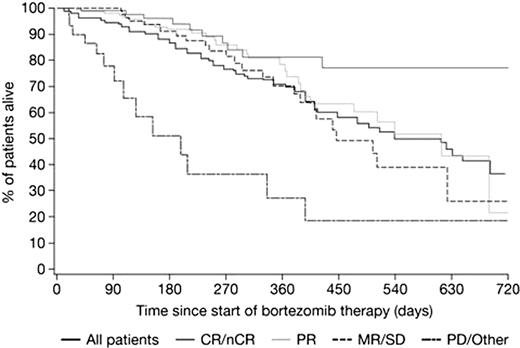
A total of 769 patients were included, 129 (16.8%) of whom achieved CR. There were no significant differences in baseline characteristics between patients who did (n=129) vs did not (n=640) achieve CR: 49.8% vs 47.3% were male, median age at baseline was 60.6 vs 62.1 years, median age at diagnosis was 57.9 vs 59.4 years, and median time since diagnosis was 2.3 vs 2.4 years. Patients with adverse prognostic factors were evenly distributed among response groups: of the patients who achieved CR, 7.8%, 55.8%, 23.3%, and 13.2% had 0, 1, 2, and 3 or more prior lines of therapy, vs 3.6%, 56.1%, 23.4%, and 15.8% of those who did not achieve CR. Similar results were seen for patients across disease stages: 14.7%, 31.0%, and 46.5% of CR patients vs 12.3%, 33.1%, and 50.2% of non-CR patients had ISS disease stage I, II, or III, respectively. Similarly, patients with comorbidities such as obesity or diabetes were evenly distributed across response groups: 6.1% and 7.1% of CR patients had obesity or diabetes, respectively, vs 4.4% and 9.6% of non-CR patients. After 4 cycles, the overall response rate (ORR) in patients who had completed 4 cycles (n=518) was 70%, including 12% complete response (CR), 16% near-CR (nCR), and 42% partial response (PR). Improved response rates were seen with prolonged therapy; after 6 cycles, the ORR in patients who had completed 6 cycles (n=321) was 82%, including 16% CR, 20% nCR, and 46% PR. As shown in the Figure, analysis of overall survival by best response at cycle 4 showed that achieving a CR/nCR is associated with significantly improved survival versus PR (p<0.0001).
Together, these results show that bortezomib, as administered in the clinical-practice setting, is highly active in relapsed/refractory MM patients, with CRs achieved among all patient subgroups. Furthermore, achievement of CR appears associated with prolonged survival and responses to bortezomib appear to improve with prolonged duration of therapy.
Delforge:Celgene: Honoraria, Speakers Bureau; Janssen-Cilag: Consultancy, Honoraria. Hulin:Janssen-Cilag: Honoraria; Celgene: Honoraria. Ganguly:Johnson & Johnson: Employment, Equity Ownership. Diels:Johnson & Johnson: Employment, Equity Ownership. Dhawan:Johnson and Johnson Research Pharmaceuticals: Employment. Dimopoulos:Millennium Pharmaceuticals, Inc.: Honoraria; Ortho-Biotech: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal