Abstract
Abstract 4909
LENALIDOMIDE IS ABLE TO RESTORE IMMUNE SYSTEM IN MULTIPLE MYELOMA PATIENTS
Annalisa Chiarenza, Nunziatina Parrinello, Piera La Cava, Eleonora Spina, Daniele Tibullo, Cesarina Giallongo, Maide Cavalli, Alessandra Romano, Paolo Fiumara, Giuseppe A. Palumbo, Francesco Di Raimondo
Multiple myeloma (MM) is a malignant plasma-cell proliferative disorder associated with dysfunctional T-cell responses. The immunomodulatory Thal derivative (IMiD) CC-5013 (lenalidomide) appears to be a promising agent for the treatment of myeloma. Although the exact antitumor mechanism of action of lenalidomide is unknown, a number of mechanisms are postulated to be responsible for it's activity (inhibition of angiogenesis, direct antiproliferative and proapoptotic effects on MM cells, suppression of pro-inflammatory cytokines, modulation of myeloma-stromal cells adhesive interactions). In addition, it has been demonstrated that lenalidomide in vitro is able to enhance T cell proliferation and to promotes ADCC.
In this study we evaluated if MM patients have a deficit of T-reg (CD4+, CD25+, and FOXP3+) and of T lymphocytes bearing CD200 (a tolerogenic molecule) and the effect of lenalidomide treatment on these parameters. In addition, we investigated whether lenalidomide could improve ex vivo the ADCC against myeloma cells.
Eight patients with previously untreated MM (median age 56 years) were treated with lenalidomide plus dexamethasone as first line therapy. Lenalidomide was given orally 25 mg daily on days 1 to 21 of a 28-day cycle. Dexamethasone was given orally 40 mg daily on days 1, 8, 15, 22 of each cycle. All patients were evaluable for response and toxicity.
Peripheral blood mononuclear cells (PBMNc) were obtained from MM patients using density gradient centrifugation (Fycoll) under sterile condictions, at the beginning of treatment and after 4 cycles of therapy. The percentage of T-reg (CD4+CD25+FOXP3+) and the expression of CD200 on T- lymphocytes were evaluated by cytometry. Twelve healthy subjects were used as control.
Moreover, PBMNc (effector cells, E) were incubated with MM cells line ARH-77 (target cells, T), previously labelled with CFDA,SE (carboxyfluorescein diacetate, succinimidyl ester) as a tracing fluorescent marker, in culture medium (RPMI-1640, 10%FCS, 1%penicillin/streptomycin) at different concentration (T/E ratio 1:20, 1:40). After 18-24 h co-colture cells were analyzed by flow cytometry and MM plasma cells cytotoxicity was calculated as the percentage of positive CFDA,SE/propidium cells. Myeloma cell viability was determined by tripan blue esclusion and apoptosis was also evaluated using Annexin V/propidium assay. Two MM patients treated in first line with a combination of Velcade, Thalidomide and Dexamethasone (VTD) were used as control and the experiments were performed in duplicate.
MM patients have a significantly lower rate of CD4+/CD25+/FOXP3+ and CD200+/CD3+ than normal (28,3±14,9/mmc and 37,8±24,7 /mmc vs 79,3±27,8 and 79,5± 48,9)(p=0,0001 and p=0,01 respectively). In our study, lenalidomide treatment resulted in an increase both of Treg cells and T-lymphocytes espressing CD200. This improvement is not statistically significant probably due to the low number of patients examined (tab I).
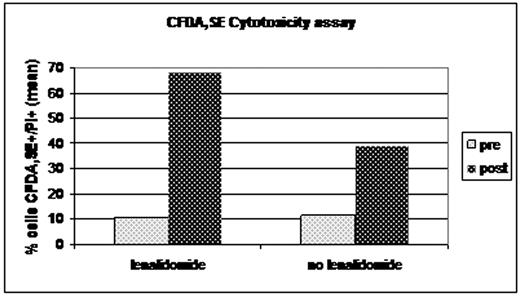
More important, we observed that PBMC derived from patients treated with lenalidomide showed an increase ability to kill a target MM cell line compared to PBMC collected at diagnosis (CFDA,SE/propidium cells 11% vs 68%). This effect was more prominent in patients treated with lenalidomide than in MM patients treated with VTD (CFDA,SE/propidium cells 12% vs 39%), Fig.1.
Our data emphasize the role of lenalidomide in modulating the endogenous tumor-specific immune response and underline the anti-myeloma activity of these new class of drugs.
| PTS (n) 8 . | CD3+CD200+ /mmc . | CD4+CD25+FOXP3+/mmc . | ||||
|---|---|---|---|---|---|---|
| Cycle | Means | Stand dev | Test t (p) | means | Stand dev | Test t (p) |
| I | 100 | 100 | ||||
| II | 426,20 | 330,64 | 0,040 | 235,40 | 154,26 | 0,027 |
| III | 364,79 | 405,98 | 0,086 | 279,25 | 146,44 | 0,013 |
| IV | 459,85 | 459,42 | 0,077 | 214,00 | 143,47 | 0,053 |
| Post IV | 376,25 | 273,33 | 0,068 | 225,25 | 163,79 | 0,123 |
| PTS (n) 8 . | CD3+CD200+ /mmc . | CD4+CD25+FOXP3+/mmc . | ||||
|---|---|---|---|---|---|---|
| Cycle | Means | Stand dev | Test t (p) | means | Stand dev | Test t (p) |
| I | 100 | 100 | ||||
| II | 426,20 | 330,64 | 0,040 | 235,40 | 154,26 | 0,027 |
| III | 364,79 | 405,98 | 0,086 | 279,25 | 146,44 | 0,013 |
| IV | 459,85 | 459,42 | 0,077 | 214,00 | 143,47 | 0,053 |
| Post IV | 376,25 | 273,33 | 0,068 | 225,25 | 163,79 | 0,123 |
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal