Abstract
Abstract 4886
Detection of high molecular weight light chain oligomers in urinary exosomes of patients with AL amyloidosis.
Exosomes are microvesicles that are part of the multivesicular body (MVB) pathway. They are created by the inward budding of the cell surface membrane and contain both surface bound membrane proteins and cytosolic proteins which can be used to identify the cell of origin. Immunoglobulin light chain amyloidosis (AL) occurs as the result of amyloid formation by the misfolding of monoclonal light chains (LC) and deposition of these amyloid fibrils in various soft tissues. This reaction requires the organization of the monoclonal LC's into protofibrils which are then weave into amyloid fibrils. This study was undertaken to determine whether urinary exosomes of glomerular origin contain intermediate species of amyloid formation.
Urine samples from patients with AL, light chain deposition disease (LCDD), multiple myeloma (MM) and monoclonal clonal gammopathy of undetermined significance (MGUS) were collected. Urinary exosomes were isolated and separated into fractions by gradient centrifugation. Western blots were performed on the urinary exosome fractions using anti-kappa or anti-lambda antibodies. Glomerular fractions were identified using antibodies directed toward podocin.
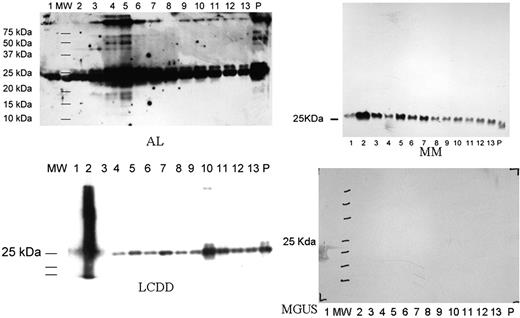
Urine samples were collected from 5 patients with AL, 2 from LCDD, 1 from MM and 1 MGUS. On the Western blot, immunoglobulin LC were seen in all exosomal fractions in patients with AL amyloidosis, LCDD, MM but not MGUS which is similar to normal controls (not shown). In patients with AL, oligomeric species were found in the highest concentrations in fraction 4 and 5 (Figure 1). Fraction 4 and 5 were also stained for podocin, a glomerular protein (not shown). The highest molecular weight species was ∼250 kd which corresponds to a LC decamer. High molecular weight species were also identified in 1 of 2 LCDD patients corresponding to a tetramer. The band was identified in fraction 10 which had polycystin-1 expression suggesting a tubular origin. No high molecular weight LC species was found in patients with MM or MGUS.
Our study found high molecular weight LC species corresponding to the intermediates involved in protofibril formation in urinary exosomes of patients with AL. Smaller (tetramer) high molecular weight LC species were also found in a patient with LCDD but not in patients with MM and MGUS. Not only were the high molecular weight LC species found exclusively in the diseases characterized by deposition of LC aggregates, they were also found in the segments of the nephron where the deposits were expected: glomerulus for AL and tubular epithelium for LCDD. This is consistent with our current understanding of the pathogenic mechanisms of these diseases. We believe urinary exosomes are a powerful tool in the study of diseases involving self-aggregation of monoclonal proteins. It has tremendous potential in both diagnostic and scientific research in this area.
Gertz:celgene: Honoraria; millenium: Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal