Abstract
Abstract 4728
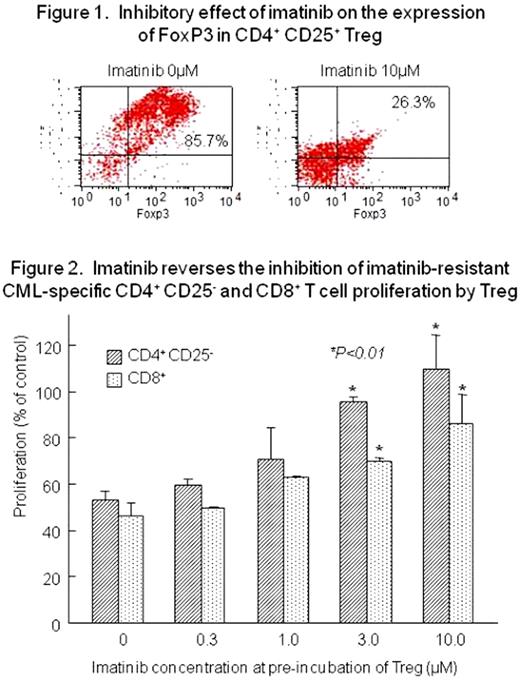
Imatinib mesylate (imatinib) has been widely used clinically for the treatment of CML and Ph-positive ALL patients with tremendous success. However, the effect is limited in advanced stage by rapid development of resistance to imatinib. Tregs play a pivotal role in peripheral tolerance and their impairment results in autoimmune disease and GVHD. Previous studies have revealed that imatinib inhibits the proliferation of several types of immune cells. However, immunomodulatory function of imatinib in CML remains to be elucidated. In the present study, we investigated whether imatinib exerts an immunosuppressive effect on naturally occurring Tregs in T cell responses to CML, especially imatinib-resistant CML. Peripheral blood mononuclear cells were obtained from 5 healthy volunteers with informed consent. CD4+CD25+ and CD4+CD25- T cells were isolated by magnetic cell sorting using human CD4+CD25+ T regulatory cell isolation kit (Miltenyi Biotec). Both CD4+CD25+ and CD4+CD25- T cells, pre-stimulated with anti-CD3 and anti-CD28 monoclonal antibody-coated beads for 2 days, were cultured with increasing doses of imatinib for 3 days and survival of the cells was evaluated by a WST1 tetrazolium assay. Viability decreased at higher concentrations (>10μM) of imatinib. There was no difference in the sensitivity to imatinib between CD4+CD25+ and CD4+CD25- T cells. To examine whether imatinib exerts an inhibitory effect on the proliferation of Tregs, freshly isolated CD4+CD25+ and CD4+CD25- T cells were cultured with varying concentrations of imatinib together with CD3 and CD28 stimulation for 3 days. The proliferation of these cells was inhibited by higher concentrations of imatinib in a similar fashion. Expression of FoxP3 in CD4+CD25+ Tregs was inhibited with 10μM imatinib by apporoximately 70% as shown in Figure 1. Because Treg has been shown to suppress immunity against cancer, a similar inhibitory effect is expected to occur in CML. This prompts us to examine immune responses to CML. For this purpose, we utilized a cell line (TM) established by us from a patient with CML in blastic crisis and another imatinib-resistant cell line (TM-G) generated by culturing TM with increasing concentrations of imatinib. CFSE-labeled CD4+CD25- and CD8+ responder cells were cultured for 5 days with irradiated autologous CD3- antigen presenting cells which were loaded with cell-lysates from either TM or TM-G in the presence or absence of Tregs which were pre-incubated with increasing concentrations of imatinib for 2 days. Proliferation of both CD4+CD25- and CD8+ responder cells were inhibited by Tregs which were not exposed to imatinib. On the other hand, Tregs which were exposed to imatinib had reduced inhibitory effects on the proliferation of responder cells depending on the concentration of imatinib as shown in Figure 2. These results demonstrate the immunomodulatory functions of imtinib in CML and imply that the use of this drug is a potent inhibitor of Tregs in cancer immunotherapy.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal