Abstract
Abstract 4119
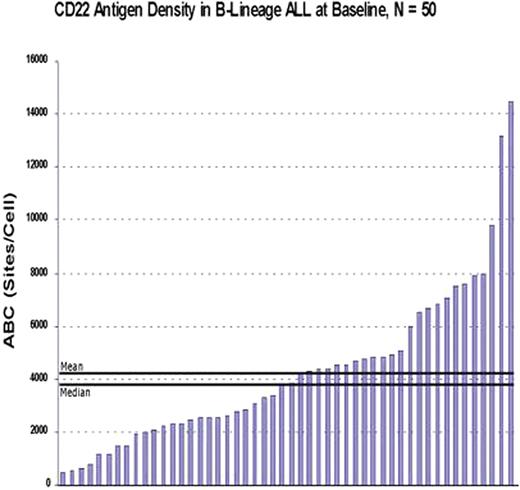
While the majority of pediatric patients with newly diagnosed B-lineage acute lymphoblastic leukemia (ALL) are cured with standard chemotherapy regimens, treatment is associated with multiple toxicities, and ALL remains the most frequent cause of cancer mortality in childhood. CD22, a B-lineage surface glycoprotein involved in B cell signaling and adhesion, is expressed in most cases of B-lineage ALL. We are conducting clinical trials of anti-CD22 immunotoxins [RFB4(dsFv)-PE38] for pediatric ALL. To assess eligibility for such targeted therapy, CD22 expression by ALL cells was studied in peripheral blood and/or bone marrow aspirate samples from 50 patients with relapsed ALL. The level of CD22 expression by ALL cells was quantitated by measuring mean anti-CD22 antibody binding per ALL cell (ABC) under saturating conditions using flow cytometry and the BD Biosciences QuantiBRITE system for fluorescence quantitation. Patients ranged in age from 3 to 22 years (median 10 years) and included 27 males and 23 females. CD22 expression was detected in all samples, and the vast majority of cases demonstrated expression of CD22 in 100% of leukemic blasts. CD22 antigen density in ALL cells varied widely among patients at baseline (range 451 - 14,519; mean 4276; median 3824; standard deviation 2976; see graph). CD22-directed immunotoxin therapy was initiated in 29 of the 50 patients, 19 of whom had samples quantitated for CD22 expression levels both before and after immunotoxin therapy. Most patients exhibited limited variation in the mean number of anti-CD22 molecules bound per ALL cell when comparing multiple specimens. In conclusion, CD22 expression varies widely in pediatric B-lineage ALL and persists despite repeated exposure to CD22-directed therapy.
(MedImmune, LLC, sponsored the clinical studies of anti-CD22 immunotoxin CAT-8015.)
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal