Abstract
Abstract 4092
Poster Board III-1027
Citokine Induced Killer (CIK) cells are a heterogenous subset of T lymphocytes sharing phenotype and functional properties with natural killer (NK) cells. CIK cells can be massively ex-vivo expanded and are capable of antitumor activity in both hematological and solid malignancies. The antitumor activity of CIK cells is classically MHC-unrestricted and mostly mediated by the interaction of CIKs' membrane receptor NKG2D with its ligands on tumor cells. The main ligand for NKG2D is MHC class I-related chain (MIC) A/B. The expression of this protein is potentially induced by pathological stimuli and is reported to be associated to some malignant phenotypes. The presence of MIC A/B on tumor targets is crucial for an effective killing by CIK cells and its accurate evaluation might help identifying the tumors, and potentially the patients, more sensitive to a CIK based immunotherapy approach.
Trastuzumab, is a humanized monoclonal antibody directed against the extracellular portion of the tyrosine kinase HER2. Although Trastuzumab has been successfully used in HER2-overexpressing breast cancer (BC) patients, resistance is a frequent problem that culminates in treatment failure. The scarsity of treatment options for resistant patients renders crucial the exploitation of new potential targets for tumor treatment.
In this context we hypothesized that MIC A/B expression might be associated with the acquisition of aggressive characteristics by BC cells, like the emersion of resistance to Trastuzumab treatment.
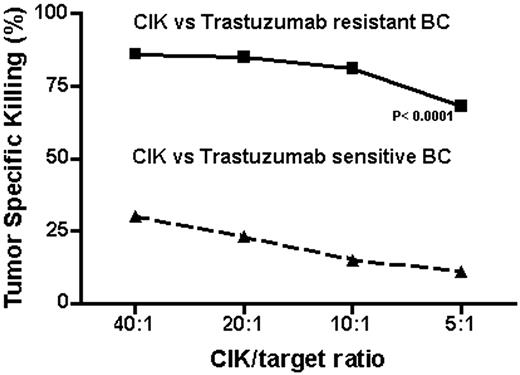
Flow-cytometry analysis showed that the expression of MIC A/B was virtually absent on HER2-overexpressing BC cells (SKBR3). These cells showed a high sensitivity to treatment with Trastuzumab in vitro, detected through cell proliferation test in soft agar and bioluminescent essays for ATP release. Similarly to what happens in the clinic, a prolonged treatment (> 6 months), determined the selection of cell clones resistant to Trastuzumab exposure. This event was found to be associated with the acquisition of a high expression of MIC A/B on cancer cells' membrane (> 95%). A high expression of MIC A/B (>95%) was confirmed also on a different BC cell line (JIMT-1) that is characterized by a primitive resistance to Trastuzumab. As hypothesized, CIK cells, generated from healthy donors, efficiently killed Trastuzumab-resistant (TR), MIC A/B positive, BC cells (86%, 85%, 81% and 68% of specific killing at a 40:1, 20:1, 10:1 and 5:1 effector/target ratio respectively) while were minimally effective against MIC A/B negative, Trastuzumab-sensitive (TS), BC cells (30%, 23%, 15% and 11% of specific killing at a 40:1, 20:1, 10:1 and 5:1 effector/target ratio respectively, p < 0.0001). The test was performed by staining target cells with Carboxyfluorescein Succinimidy ester (CFSE) and co-culturing them with CIKs at different ratios; data were analyzed after 4 and 12 hours by flow cytometry (Fig.1).
Our data describe for the first time the association of MIC A/B upregulation on HER2 overexpressing BC cells and Trastuzumab resistance. This biological event induces the susceptibility of cancer cells to tumor killing operated by CIK cells. If confirmed on breast cancer tissues, our data might provide a rationale for CIK based immunotherapy in HER2 overexpressing breast cancer patients developing clinical resistance to Trastuzumab. Additional studies will investigate the possible molecular links between Trastuzumab resistance and MIC A/B expression.
CIK vs Trastuzumab sensitive BC
CIK vs Trastuzumab resistant BC
P< 0.0001
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal