Abstract
Abstract 408
R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone) is the current standard of care for the treatment of diffuse large B cell lymphoma (DLBCL). Doxorubicin has an integral role but is associated with significant toxicity. The omission of doxorubicin in patients who are frail, have poor baseline cardiac function or who have previously received anthracycline therapy may compromise outcome. However, the recent introduction of rituximab may allow for improved outcomes without the use of doxorubicin. We assessed the efficacy of substituting etoposide for doxorubicin for patients with a contraindication to anthracyclines.
Treatment guidelines in British Columbia (BC) recommend the substitution of etoposide for doxorubicin in standard dose R-CHOP for patients with DLBCL who have a contraindication to anthracyclines (R-CEOP). Etoposide is administered at a dose of 50 mg/m2 IV on day 1 and 100 mg/m2 PO on days 2 and 3 of each cycle. Patients with limited stage disease receive 3-4 cycles of chemotherapy with or without radiation therapy; patients with advanced stage disease receive 6 cycles of chemotherapy. We performed a retrospective population-based analysis using the BC Cancer Agency Lymphoid Cancer and pharmacy databases and included all patients with newly diagnosed DLBCL who were treated with curative intent with R-CEOP from December 2000 to March 2009. Patients who began treatment with R-CHOP but then switched to R-CEOP (due to intolerance or because a maximum threshold of anthracycline dosing was reached) were also included in the analysis if R-CEOP was administered for more than 50% of cycles delivered. Outcome of this population was compared with a 2:1 control group treated with R-CHOP in the same time period and matched for age, clinical stage and International Prognostic Index (IPI). End points were time to progression (TTP) and overall survival (OS).
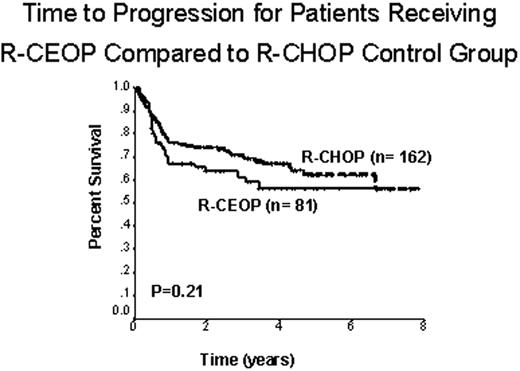
We identified 81 patients treated with R-CEOP who met the stated inclusion criteria. Clinical characteristics were as follows: median age 73 y (range 34-93), 55 (68%) male, 15 (18%) limited stage and 66 (82%) advanced stage. Adverse prognostic factors according to the IPI were as follows: 85% age > 60 y, 58% stage III/IV, 31% PS > 1, 43% elevated LDH, and 12% > 1 extranodal site. IPI score: 19 (23%) low risk, 33 (41%) low-intermediate, 18 (22%) high-intermediate, and 11 (14%) high risk. Reasons for etoposide substitution were: 71 (88%) cardiac contraindication, 7 (9%) prior exposure to anthracyclines and 3 (4%) other co-morbidities. 72 (89%) patients received rituximab as part of the treatment, 25 (31%) received partial treatment with R-CHOP and then switched to R-CEOP and 56 (69%) received no anthracycline therapy. The control group included 162 randomly chosen patients matched for age, stage and IPI score and treated with R-CHOP. At a median follow-up of 28 months (range 3-86), 33/81 (41%) R-CEOP patients had died (23 with lymphoma, 3 treatment toxicity, 7 unrelated causes). The 5-year TTP was similar for patients treated with R-CEOP compared to patients in the R-CHOP control group, 57% versus 62%, respectively (p=0.21). (Fig. 1) The 5-y OS was lower for patients receiving R-CEOP compared with the R-CHOP control group (49% versus 64%, p=0.02), reflecting the underlying co-morbidities and frailty of this population. Interestingly, the 5-y TTP and 5-y OS rates were similar for patients in the R-CEOP cohort who had received partial treatment with an anthracycline and those who had received no anthracycline treatment (p=0.49 and p=0.77, respectively).
A significant proportion of patients with DLBCL who are unable to receive anthracycline-based therapy can be cured with the etoposide-substituted regimen R-CEOP. R-CEOP is well tolerated even by patients with numerous co-morbidities. Moreover, the outcome of these patients does not appear to be significantly different compared to a similar population treated with standard R-CHOP.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal