Abstract
Abstract 4069
Poster Board III-1004
Transfused patients with β-thal major are known to experience clinical consequences of cardiac iron overload despite the widespread use of iron chelation therapy. Approximately 71% of patients will suffer cardiomyopathy, congestive heart failure (CHF) and death. Previous trials have confirmed the efficacy of deferasirox (Exjade®) in removing cardiac iron in patients with β-thal major. This ongoing study evaluates the effects of deferasirox on cardiac iron and left ventricular ejection fraction (LVEF) in patients with β-thal major in a prospective, single-arm, multi-center trial using cardiac MRI T2*. All patients have completed 18 months of therapy and we also report preliminary results from 24 months.
28 patients were enrolled at four US centers. Entry criteria included MRI evidence of cardiac iron (T2* <20 ms) and normal LVEF (≥56%). Deferasirox was administered at 30–40 mg/kg/day for 18 months. Following core study completion (18 months), patients could continue treatment for an additional 6 months if their 18-month cardiac T2* was <20 ms and they demonstrated ≥25% improvement in cardiac T2* or LIC from baseline. Serum ferritin (SF) was assessed monthly. Liver iron concentration (LIC), cardiac T2* and LVEF were assessed by MRI every 6 months. Serum creatinine (SCr), biochemical and hematological status were also monitored. All results are reported as mean ± SE (range) unless otherwise stated.
Baseline: All 26 evaluable patients (7 M/19 F; aged 10–44 years) received ≥150 lifetime transfusions. SF was 4307 ± 613 ng/mL (312–12,655), cardiac T2* was 9.5 ± 0.8 ms (1.8–16.1), LIC was 20.6 ± 3.15 mg Fe/g dry weight (dw; 3.6–62.3) and LVEF was 61.8 ± 0.8%.
At the time of analysis, 22 and 9 patients had 18- and 24-month evaluations, respectively. Six patients discontinued the core trial due to patient decision (n=2), adverse events (AEs; n=2) or abnormal lab tests (n=2). Two of these patients died after discontinuing; the first enrolled with markedly elevated baseline cardiac iron (T2* = 1.8 ms) and died secondary to CHF. The second patient withdrew due to an AE and died 2 months later due to sepsis and multi-organ failure.
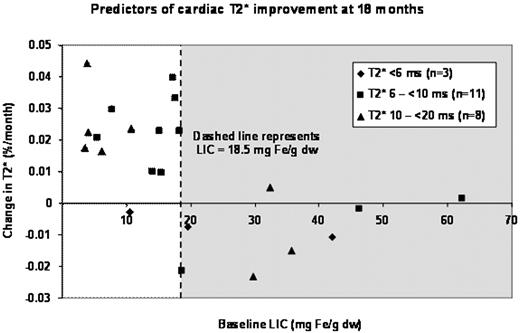
18-month results: At 18 months, 10/22 patients were on 40 mg/kg/day. The mean improvement in cardiac T2* from baseline in all patients was 2.2 ms (22%; P=0.016), with 13 patients improving, four remaining stable (T2* change <10%) and five worsening. Baseline LIC was a powerful predictor of response (Figure); cardiac T2* in 14 patients with LIC <18.5 mg Fe/g dw improved 2.2% per month, with 13/14 patients showing large improvements and one patient remaining stable. In contrast, in eight patients with LIC >18.5 mg Fe/g dw, mean T2* worsened 1.4% per month (P<0.0001); three patients remained stable and five worsened significantly. Improvements in cardiac iron were correlated with changes in LIC (r2 = 0.27, P=0.013). In general, initial T2* did not predict therapeutic response, although all three patients with T2* <6 ms increased their cardiac iron. LIC decreased 4.1 mg Fe/g dw over the study interval (P=0.003). LVEF remained stable.
24-month results: At 24 months, 7/9 patients were on 40 mg/kg/day. Relative to the 18-month time-point, 8/9 patients (89%) increased their cardiac T2*, with a mean improvement of 2.7% per month. Mean LIC, SF and LVEF were unchanged over the extension. Safety parameters from patients treated with 30–40 mg/kg/day deferasirox (n=25) were in line with previous studies at 20–30 mg/kg/day.
Deferasirox monotherapy resulted in statistically significant improvements in cardiac and hepatic iron after 18 months. Baseline LIC <18.5 mg Fe/g dw was a strong predictor of favorable response. LVEF remained stable during the study. Patients in the extension (18–24 months) improved their cardiac T2* without further improvements in LIC or SF. Deferasirox monotherapy at 30–40 mg/kg/day provides good cardiac chelation in patients with moderate cardiac and liver iron burdens. More aggressive therapy is warranted for more severe iron overload.
Wood:Novartis: Research Funding. Thompson:Novartis: Research Funding. Paley:Novartis Pharmaceuticals: Employment, Equity Ownership. Glynos:Novartis Pharmaceuticals: Employment. Kang:Novartis Pharmaceuticals: Employment, Equity Ownership. Giardina:Novartis: Research Funding, Speakers Bureau. Harmatz:Ferrokin: Membership on an entity's Board of Directors or advisory committees; Apotex: Membership on an entity's Board of Directors or advisory committees. Coates:Hope Pharma: Consultancy, Research Funding; Sangart Pharma: Consultancy, Honoraria; Novartis: Consultancy, Honoraria, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal