Abstract
Abstract 4057
Poster Board III-992
Iron overload is a serious complication found in thalassemia patients requiring regular transfusion. Subcutaneous infusion of desferrioxamine for 8-12 hours/day five days per week creates constraint and incomplete compliance especially pediatric patients.
The purpose of this study is to evaluate the effectiveness of daily 50-75 mg/kg of locally-produced oral deferiprone (GPO-L-One from Thai Governmental Pharmaceutical Organization) combined with 40 mg/kg of subcutaneous infusion of desferrioxamine two days per week in pediatric patients with transfusion-dependent thalassemia disease.
49 patients with homozygous beta-thalassemia disease (n=7) and beta-thalassemia/Hb E disease (n=42) with a median age of 10 years were enrolled in the study. Splenectomy was performed in four and eight patients with homozygous beta-thalassemia disease and beta-thalassemia/Hb E disease, respectively. They required regular packed red cell transfusion of 15-20 ml/kg every 3-4 weeks to maintain the pre-transfusion hematocrit at 27%. The median level of ferritin was 2,907 ng/mL (interquartile range 2,419-3,686 ng/mL). The clinical manifestation and complications of iron chelation were closely monitored. Complete blood counts, BUN, creatinine, liver profile, serum ferritin and urine examination were checked monthly. Eight to ten hours of subcutaneous infusion of desferrioxamine was given using an infusion pump. The patients initially received deferiprone at the dose of 50 mg/kg and increased to 75 mg/kg at the fifth month if the declination of serum ferritin was less than 15% of the initial level.
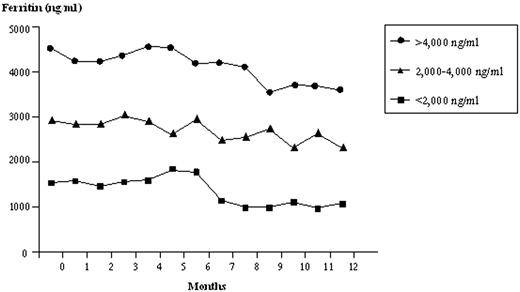
The patients were divided into three groups according to their serum ferritin levels: group 1, n=9, serum ferritin was more than 4,000 ng/ml; group 2, n=31, serum ferritin between 2,000 and 4,000 ng/ml and group 3, n=9, serum ferritin was less than 2,000 ng/ml. The median required dose of deferiprone in groups 1 and 2 (75 mg/kg) was significantly higher than that of group 3 (50 mg/kg) with a p value of 0.0001. The serum ferritin levels were gradually decreased during one-year studied period as shown in Figure 1. Ten minimal side effects of deferiprone occurred mostly in the first four months of treatment among 8 out of 49 patients including neutropenia less than 1,500/mcl (n=4); platelet counts less than 150,000/mcl (n=2); slightly high serum creatinine (n=2); serum ALT of 292 unit/L (n=1) and proteinuria of 10 mg/m2/h with normalized serum creatinine (n=1). No patient had agranulocytosis. These patients received deferiprone at the dose of 50 mg/kg in 6 cases and 75 mg/kg in 2 cases. All the abnormal laboratory investigations were transient and became normalized within one month. However, deferiprone was discontinued in four patients with ANC of 1,406/mcl, ANC 1,461/mcl, platelet counts of 130,000/mcl and proteinuria.
The preliminary study of using locally-produced deferiprone combined with two-day infusion of deferrioxamine is tolerable and creates satisfaction to the studied patients.
The median levels of serum ferritin during the treatment period according to the initial levels of serum ferritin.
The median levels of serum ferritin during the treatment period according to the initial levels of serum ferritin.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal