Abstract
Abstract 403
Mantle Cell Lymphoma (MCL) is an uncommon histology of non-Hodgkin's lymphoma (NHL) with an unfavorable prognosis for which optimal initial therapy has not been clearly defined. Despite a number of single center studies and uncontrolled trials examining first-line therapy options in MCL, no randomized clinical trials or observational studies have directly compared initial therapeutic options in a single cohort of patients. The role of aggressive induction therapy versus sequential standard chemotherapy remains uncertain, particularly in younger patients. We therefore used the NCCN NHL Outcomes Database to compare R-HyperCVAD, R-CHOP followed by HDT/ASCR and R-CHOP alone as first-line therapy. Our endpoints were progression-free survival (PFS) and overall survival (OS).
The NCCN Non-Hodgkin's Lymphoma Outcomes Database is a prospective cohort study collecting comprehensive clinical, treatment, and outcome data for patients seen at 7 participating NCCN centers. Overall, 229 patients <65 years old with newly diagnosed MCL presented at NCCN institutions between August 2000 and February 2009. Patients were excluded if they (1) were enrolled in clinical trial (n=27), (2) did not receive Rituximab therapy (n=27), (3) did not receive either R-HyperCVAD or R-CHOP induction therapy (n=10), or (4) received both R-CHOP and R-HyperCVAD as induction (n=9). Induction therapy was determined as the initial chemo-immunotherapy received within 180 days of diagnosis. HDT/ASCR consolidation was defined as a transplant after achieving remission. In the R-CHOP+ HDT/ASCR group, ASCR was initiated within 100 days of induction therapy for 90% of patients. The maximum time from induction to ASCR was 172 days. In total, 156 patients were included in the final analysis. Median follow-up was 30 months.
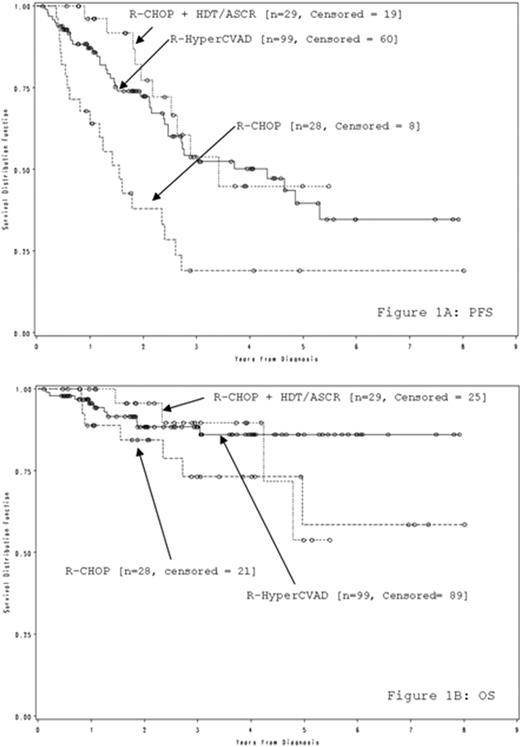
Overall, 28 (18%) patients received R-CHOP alone, 29 (19%) received R-CHOP+HDT/ASCR, and 99 (63%) received R-HyperCVAD. No significant differences were observed between therapy groups with regards to co-morbidity (p=0.419), ECOG performance status (p=0.216), B symptoms (p=0.685), bulky disease (p=0.647), IPI risk group (p=0.247), or bone marrow involvement (p=0.651). No difference in PFS (p=0.546) was observed between the R-HyperCVAD and R-CHOP+HDT/ASCR arm (figure 1a). R-CHOP without consolidation had significantly poorer PFS than both R-HyperCVAD (p=0.001) and R-CHOP+HDT/ASCR (p=0.001). No significant differences in OS were observed between the three groups. However, there was a strong trend favoring R-HyperCVAD over R-CHOP (p=0.082, figure 1b).
R-CHOP was inferior to both R-HyperCVAD and R-CHOP+HDT/ASCR, which had equal PFS and OS in patients with de novo mantle cell lymphoma. Even with these aggressive induction regimens in younger patient populations, the median PFS in these cohorts was only 3 years. Future trials should focus on incorporating novel agents rather than comparing efficacy of current suboptimal regimens.
Czuczman:NCCN: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Nademanee:Genzyme Corporation: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Blayney:American Society of Clinical Oncology: Membership on an entity's Board of Directors or advisory committees; BlueCross Blue Shield of Michigan: Research Funding; NCCN: Honoraria; University of Michigan Health System: Employment. Friedberg:Genentech: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal