Abstract
Abstract 3944
Poster Board III-880
MCL is a clinically aggressive lymphoma characterized by frequent relapses and short survival. Clinical behavior and response to therapy vary considerably and are often unpredictable based on clinical factors. Prior gene and protein expression studies have demonstrated proliferation and TP53 expression as biological mechanisms that strongly influence survival (Rosenwald Cancer Cell 2003 & Katzenberger Blood 2006 and Louie Blood 1995). Recent studies have highlighted the role of the microenvironment in follicular lymphoma (FL) including numbers of lymphoma-associated-macrophages (LAM), as important prognostic factors (Dave NEJM 2004 & Farinha Blood 2005). Within most tumors, macrophages display an M2 phenotype that promotes tumor growth and angiogenesis. In MCL, the clinical impact of LAM is unknown. Our aim was to assess the prognostic impact of LAM in MCL patients from a single institution experience using tissue microarrays (TMA), immunohistochemistry and clinical correlates.
A TMA block was built with duplicate 0.6mm cores of paraffin-embedded formalin-fixed diagnostic biopsies from 185 patients treated at the BC Cancer Agency (1983-2004). A subset of cases was also analyzed for the presence of the t(11;14) by FISH. Primary and subsequent therapy included multiple regimens: single or multi-agent chemotherapy with or without rituximab, stem cell transplant, radiation and therapeutic splenectomy. Standard immunohistochemistry was performed with CD20, CCND1, CD68, CD163 (an M2 marker), CD34, TP53 and Ki67. TP53 and Ki67 IHC were scored using image analysis (VIAS). Univariate and multivariate correlates of overall survival (OS) were determined using SPSS software.
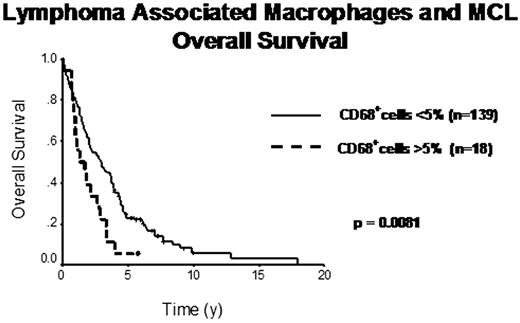
The median follow-up of living patients was 5.75 years. The IPI predicted OS (p=0.0013). Numbers of cases with interpretable staining varied for the different biomarkers (range 157 to 180). All 185 cases were either CCND1 positive and/or t(11;14) FISH positive. Both proliferation (quartiles of Ki67+ cells) and TP53 (dichotomized based on cut-off >40%) were significant predictors of OS independent of IPI (OS, p=0.0002). Cases showing more than 5% infiltrating CD68+ cells (18/185) had a 4-y OS of 5% vs. 35% (p=0.0081). These CD68+ cases significantly correlated with 12 cases showing more than 5% infiltrating CD163+ cells (x2=0.001), which also significantly influenced OS (p=0.008). 17 cases had increased microvessel density (MVD), defined by CD34+ cells, which correlated with inferior OS (p=0.02). Interestingly, cases with increased MVD often had >5% CD163+ cells (x2=0.05), but not increased CD68+ cells. Multivariate analysis including IPI, TP53, Ki67, CD68, CD163 and CD34, showed IPI (RR=1.8, 95%CI=1.0-3.1, p=0.047), TP53 (RR=2.3, 95%CI=1.3-4.3, p=0.006), Ki67 (RR=2.2, 95%CI=1.2-4.2, p=0.012) and CD68 (RR=2.1, 95%CI=1.1-3.8, p=0.022) to be independent predictors of OS. Finally, in the 27 patients who received rituximab at some time during their disease course, increased CD68+ cells did not significantly affect OS (p=0.1), suggesting that, similar to FL, the prognostic effect of LAM may be abolished by rituximab.
The number of LAMs is an important prognostic factor in MCL, independent of clinical parameters, proliferation and TP53 expression. Expression of CD163, described as an M2 polarized macrophages, also predicts survival and correlates with increased angiogenesis. These preliminary data suggest that the use of rituximab may abrogate the prognostic impact of LAM in MCL. In summary, similar to other lymphoma subtypes, the microenvironment is important in MCL biology and prognosis and may be an important new target for therapy in this aggressive disease.
Connors:Roche Canada (F Hoffmann-La Roche): Research Funding. Gascoyne:Roche Canada, Genentech, Lilly, Millennium : Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal