Abstract
Abstract 3853
Poster Board III-789
Light chain (AL) amyloidosis is a clonal plasma cell disorder characterized by progressive multiorgan deposition of amyloid fibrils derived from immunoglobulin light chains. Treatment strategies for AL have typically targeted the clonal plasma cell and have paralleled that for myeloma, with recent clinical trials evaluating the role of novel agents such as immune modulatory drugs (IMiDs). Lenalidomide has activity in patients with AL, and given the synergy seen with alkylating agents, we designed a phase 2 clinical trial of cyclophosphamide added to lenalidomide and dexamethasone.
Patients (Pts) with newly diagnosed or previously treated AL, with adequate hematological and organ function, were enrolled between December 2007 and November 2008. Treatment consisted of 28-day cycles with Lenalidomide at 15 mg daily (days 1-21), oral cyclophosphamide 300 mg (days 1, 8, 15) and dexamethasone 40 mg weekly. All patients received aspirin for thromboprophylaxis. Hematological and organ responses were assessed using conventional response criteria.
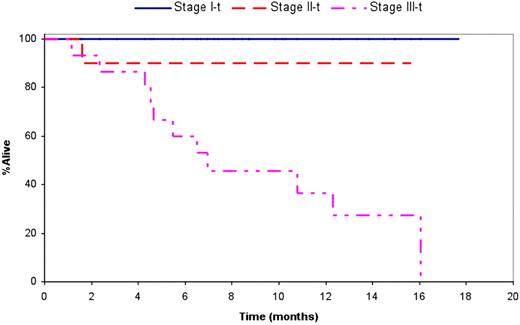
Thirty-five pts, 11 (31%) previously treated, with a median age of 64 yrs (range 44-82) were enrolled. Median time from diagnosis to trial enrollment was 1.6 mos (range; 0.1- 129) and 23 (66%) were alive at the time of analysis with a median follow up of 8.7 mos (range; 1.5-17.6). Three or more organs were involved in 10 (28%) pts with cardiac and renal involvement present in 22 (63%) and 27 (77%) respectively. Fifteen pts (44%) had advanced stage III disease by the troponin/ NT-ProBNP staging system. Seven (20%) pts had previous stem cell transplant, one pt had thalidomide and others had melphalan, dexamethasone or a combination of the two. The median duration on study was 6.5 months and the median number of cycles administered was 6, with 27 (77%) patients completing at least 3 cycles of therapy. Thirteen patients still remain on study, with the most common reason for study discontinuation being toxicity (10 pts, 45%). There were 15 (43%) and 24 (69%) episodes of Grade 3 or 4 hematologic and non-hematologic adverse events respectively. The most common non-hematologic toxicities seen were fatigue, edema, constipation and rash. Six patients died while on study due to sepsis (1), multiorgan failure (1), arrhythmia (1), or heart failure (3). The overall hematologic response rate was 60% (21 pts; CR (2), VGPR (10) and PR (9)); 79% for stage I and II pts and 40% for stage III patients. Among those patients receiving at least four cycles, the response rate was 20/23 (87%); 2CR, 10 VGPR and 8 PR. At least one organ response was documented in 8 (24%) patients. The median overall survival (OS) for all enrolled patients is 16.1 mos (95% CI; 10.8-NR). The median OS was not reached for stage I and stage II patients, and was 7 mos (95% CI; 4.6 - 16.1) for stage III pts. The median time to hematologic progression was 16.1 mos and to hematologic or organ progression was 6.7 mos (95% CI; 6.3, 16.1). The median OS among those with a hematologic response was 16.1 mos, and not reached among the group with a VGPR or better. Among the 31 patients with measurable FLC, those (n=24) with a ≥50% decrease in the difference between light chains had superior OS compared to the rest (16.1 mos vs. 4.3 mos). The six patients who died on study all had cardiac involvement and significantly higher NT-ProBNP/ BNP compared to the rest.
The combination of cyclophosphamide, lenalidomide and dexamethasone has significant activity in patients with light chain amyloidosis. Toxicity is manageable with dose reductions, but patients with advanced cardiac involvement are at a higher risk for toxicity and early discontinuation of therapy. Future trials with this combination should compare this regimen with other treatment approaches in selected patients, as well as pursue a dose-adapted strategy in those with advanced cardiac involvement.
Kumar:CELGENE: Research Funding; MILLENNIUM: Research Funding; BAYER: Research Funding; GENZYME: Research Funding; NOVARTIS: Research Funding. Gertz:celgene: Honoraria; millenium: Honoraria, Membership on an entity's Board of Directors or advisory committees. Dispenzieri:Celgene: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal