Abstract
Abstract 3809
Poster Board III-745
Epigenetic therapy with hypomethylating agents has recently been approved for the treatment of myelodysplastic syndromes (MDS) in South Korea and Argentina. Chronic Myelomonocytic Leukemia (CMML) is a hybrid disorder characterized by myeloid proliferation and erythroid-megakaryocytic dysplasia. Subgroups analysis (Steensma D et al. JCO.2008.19) and open-label studies (Aribi A, Cancer 2007;109:713-7) have reported that decitabine (DACOGEN, Janssen Cilag Farmaceutica S.A. and Eisai Inc.) is effective in the management of CMML.
To describe the clinical and hematological improvement with decitabine among patients with CMML on a “real world program”.
We enrolled patients with CMML, who received decitabine at different centers of South Korea and Argentina, between July 2007 and June 2009. A report prepared ad hoc was completed. We took into account WHO classification, as well as performance status by ECOG, co-morbidities, previous treatments and IWG 2006 criteria. Efficacy was evaluated with at least 2 cycles. Inclusion criteria were ≥18 years of age and confirmed diagnosis of CMML type 1 or type 2. Exclusion criteria were diagnosis of acute myeloid leukemia (AML) or other progressive malignant disease. Patients with prior therapy were not excluded. All patients received decitabine 20 mg/m2 IV over 1 hour once daily for 5 consecutive days repeating every 4 weeks. We evaluated the overall improvement rate (complete response + marrow complete response + partial response + hematologic improvement) and rate of stable disease or better.
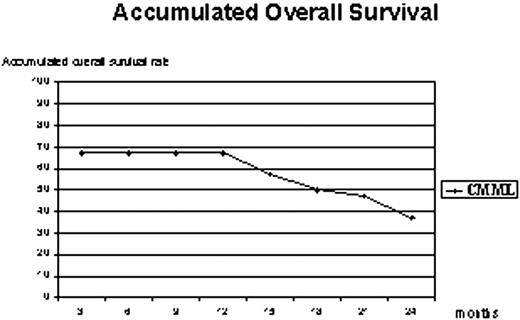
We analyzed 26 CMML patients, Type-1: 65% and Type-2: 35%, median age 61 (R 23-82), male: 81%, all patients were BCR/ABL negative, and 23% had proliferative features with WBC >13000/mm3 and splenomegaly at the time of diagnosis. Karyotype was normal (n=19), isolated -7/7q- (n=2), +8 (n=1), del3q/der3 (n=1), tY/1 (n=1), complex (n=1) and no metaphases (n=1). The median interval from diagnosis to treatment was 8 months (R 0-35); Eight patients received previous chemotherapy: low dose (n=4), high dose (n=2) or bone marrow transplant (n=2); and the median number of cycles received was 5 (R 1-13). Clinical and Hematological response are summarized in Table. Most of the patients remained alive during the first year of follow-up. The accumulated overall survival curve showed a plateau that lasted until the end of the first year; afterwards it progressively decreased (Graphic). Two patients received allogeneic stem cell transplant without additional toxicity.
Decitabine demonstrated a remarkable activity (58%) in CMML with an accumulated overall survival of 37% at 2 years of follow-up. This treatment allowed patients to be transplanted in a better condition.
| Treatment response, n (%) . | CMML (N=26) . |
|---|---|
| Complete Response (CR) | 6 (23%) |
| Partial Remission (PR) | 0 |
| Marrow complete response (mCR) | 1 (4%) |
| Hematologic improvement (HI) | 2 (8%) |
| Stable Disease (SD) | 6 (23%) |
| Overall Improvement Rate (CR+PR+mCR+HI) | 9 (35%) |
| Rate of stable disease or better | 15 (58%) |
| Accumulated Overall Survival (%) at 2 years | 37% |
| AML Development, n (%) | 4 (15%) |
| Failure: (Progressive Disease or Death) | 11 (42%) |
| Treatment response, n (%) . | CMML (N=26) . |
|---|---|
| Complete Response (CR) | 6 (23%) |
| Partial Remission (PR) | 0 |
| Marrow complete response (mCR) | 1 (4%) |
| Hematologic improvement (HI) | 2 (8%) |
| Stable Disease (SD) | 6 (23%) |
| Overall Improvement Rate (CR+PR+mCR+HI) | 9 (35%) |
| Rate of stable disease or better | 15 (58%) |
| Accumulated Overall Survival (%) at 2 years | 37% |
| AML Development, n (%) | 4 (15%) |
| Failure: (Progressive Disease or Death) | 11 (42%) |
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal