Abstract
Abstract 3758
Poster Board III-694
The combination of Bortezomib (B) and Rituximab (R) has been shown in vitro synergistic apoptosis and enhanced NFkB depletion in Mantle Cell Lymphoma (MCL) and Marginal Zone Lymphoma (MZL) cells. Rituximab in combination with Bortezomib is well tolerated without overlapping toxicities. Our study was aimed to evaluate safety and efficacy of the association of Rituximab and Bortezomib in relapsed/refractory indolent non follicular and mantle cell lymphoma patients not eligible to high dose chemotherapy and ASCT. The study was designed according to Simon's two stage Optimal Design and the primary endpoint was the achievement of an Overall Response Rate (ORR) > 40%.
From September 2006 to March 2008, 54 patients were enrolled into the study. Histology was centrally reviewed in all cases: 49 diagnosis fulfilled the inclusion criteria and were evaluable for the analysis. Clinical characteristics were as follows: 28 males and 21 females; median age 68 years (range 50-74); 16 lymphocytic/lymphoplasmacytic (LL), 8 MZL and 25 MCL; seven stage I-II disease, nine III and 33 IV; 33 were BM positive; according to IPI 36 patients were at low-intermediate risk and 18 at intermediate-high/high risk; 11 patients had 1 and 38 > 2 prior lines of chemotherapy; 15 were R-naïve patients and 34 R-pretreated, 21 patients had refractory disease (<1 yr from the last therapy) and 28 were in relapse (> 1 yr). The treatment plan was: one course of 4 weekly doses of Rituximab (375 mg/sqm) and Bortezomib (1.6 mg/sqm IV bolus) followed by 2 courses of 4 weekly IV bolus of B (1.6 mg/sqm) as single agent. Responding (CR+ PR) and stable disease patients were planned to be given three further courses with the same schedule.
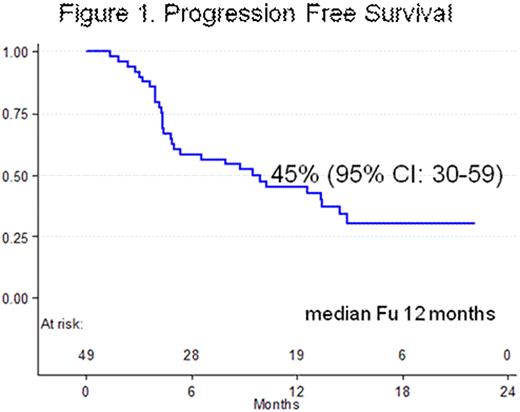
ORR was 26/49 (53%). Responses were as follows: 13 CR (26.5%) and 13 PR (26.5%). ORR by histology was: 6/16 (37.5%) in LL, 4/8 (50%) in MZL and 16/25 (64%) in MCL respectively. Pretreatment with Rituximab did not adversely affect the ORR: 21/34 (62%) in R-pretreated patients and 5/15 (33%) in R-naïve patients (p .06). ORR was higher in relapsed patients compared with refractory ones: 18/28 (64%) and 8/21 (38%) (p .06). With a median follow-up of one year, Overall Survival (OS) was 93% (95%CI: 79.7-97.9) and 1-year Progression free survival (PFS) was 45% (95%CI: 30-59) (Figure 1). A total of 233 courses were delivered with a median of 4.7 courses per patient. Twenty-eight patients completed the treatment plan and 21 were withdrawn from the study because of: 17 PD during treatment and 4 AE (1 concomitant gastric neoplasia, 1 neurotoxicity grade II, 1 pleural effusion and 1 toxic death due to interstitial pneumonia). Grade 3-4 CTC haematological toxicity was rare with neutropenia in 5% of the courses and thrombocytopenia in <2%. Grade 3-4 CTC cumulative non hematological toxicity was observed in 4.7% of all courses delivered. The most frequent of these were: neurotoxicity grade II in 13 patients and grade III in 5, with complete recover or return to grade I in all of them but one; infections were detected in 3 patients (pneumonia), constipation grade III in 2 and diarrhoea grade > III in 5.
This study suggests that the combination of Rituximab and Bortezomib is feasible and effective in relapsed/refractory indolent non follicular lymphoma and MCL. This treatment combination is a good therapeutic option without chemotherapy mainly in MCL and MZL also in Rituximab pretreated patients. Our data suggest a promising PFS in this subset of heavily pretreated patients. The combination of Rituximab plus Bortezomib should be explored in further and larger studies.
Progression Free Survival
Vitolo:Roche: lecture fees. Off Label Use: Bortezomib was provided free by Jansen-Cilag who gave a research grant to support the study.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal