Abstract
Abstract 3741
Poster Board III-677
LNH98.5 was the first randomized study with the addition of rituximab to CHOP in patients with diffuse large B-cell lymphoma. 399 patients were randomized, 197 in CHOP arm and 202 in R-CHOP arm. Patients were aged between 60 and 80 years (median 70 years), had disease stage II to IV, and no contra-indication to one of the drugs. 60% had poor risk lymphoma according to IPI. Response to treatment and early survival analyses were previously presented with 2 years and 5 years median follow-up (NEJM 2002;346:235 and JCO 2005;23:4117).
With a median follow-up of 10 years, median age of surviving patients is 78 years, oldest patient being 91 years old. Only 4 patients were lost for follow-up, defined as not seen during the last 18 months, at 5, 7, 8, and 8 years. No event was observed in 105 of the 399 patients, 37 (19%) in CHOP arm and 68 (34%) in R-CHOP arm. Relapse was observed in 73 (59%) and 51 (34%) of CR patients, and death without progression in 16 and 33 patients, respectively. Death occurred in 71% and 56% of the patients, most of them from disease progression but 21 and 20 cancers were observed, representing half of the deaths without progression. Most frequent cancers were colon and lung; two MDS were observed in CHOP arm and one AML in R-CHOP arm. One patient with CHOP presented a multiple myeloma 10 years after DLBCL.
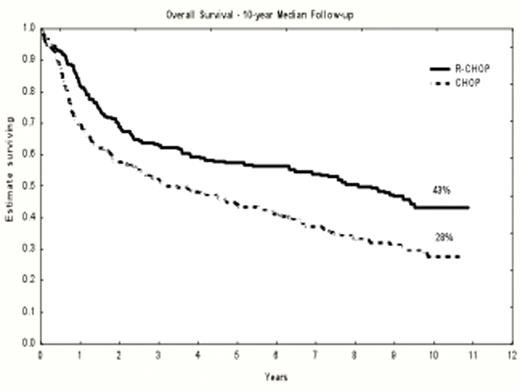
During the last 3 years, 10 additional patients relapsed, 4 in CHOP arm and 6 in R-CHOP arm, these late relapses representing 4% of CR patients. Median overall survival was 37 months in CHOP arm and 7 y 9 m in R-CHOP arm with 10-y survival of 28% and 43%, respectively (p<0.001). Median survival from progression was 8 months in both arms.
This analysis showed that the benefit of combining rituximab with CHOP chemotherapy persists with a median follow-up of 10 years and that over 40% of elderly patients are alive 10 years later confirming these patients could express long-term survival if treated like younger patients. However, late relapses do occur and new strategies should be developed to prolong the response of these patients.
Coiffier:Roche: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Gisselbrecht:Roche: Research Funding, Speakers Bureau. Bosly:Roche: Research Funding, Speakers Bureau. Herbrecht:Roche: Research Funding. Bouabdallah:Roche: Research Funding. Morel:Roche: Research Funding. Van Den Neste:Roche: Research Funding. Bordessoule:Roche: Research Funding. Haioun:Roche: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Tilly:Roche: Research Funding, Speakers Bureau. Salles:Roche: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal