Abstract
Abstract 3540
Poster Board III-477
A strategy that can induce stable mixed chimerism across human leukocyte antigen (HLA) barriers would be beneficial in extending the application of hematopoietic stem cell transplantation (HSCT) to patients with severe sickle cell disease (SCD) who are in need of this potentially curative procedure. Indeed, we have recently demonstrated the feasibility of an HLA-matched sibling protocol employing low dose total body irradiation (TBI, 300cGy), the lymphocyte depleting agent alemtuzumab, and sirolimus to reverse the phenotype with minimal side effects. Due to the lack of HLA-matched siblings in the majority of patients, our goal is to develop a safe haploidentical regimen. In this work, we focused on determining optimal postgrafting immunosuppression and examined sirolimus and post-transplant cyclophosphamide (PT-cy), agents known to induce transplantation tolerance. To determine the optimal sequence for combining these drugs and whether this combination is synergistic in promoting stable donor chimerism despite the antiproliferative effects of sirolimus, we used a mismatched murine model with BalbC donors and C57Bl6 recipients. Twenty-five to 40 recipient mice received 200cGy TBI and PT-cy (200mg/kg intraperitoneally (IP) 2 days post transplant) with or without sirolimus (3mg/kg IP) for 14 to 30 days starting 1 day before or 4, 6, or 10 days post transplant. We found that in contrast to sirolimus or PT-cy alone, the combination of PT-cy and a limited course of sirolimus resulted in stable mixed chimerism: all mice that received PT-cy and sirolimus starting between 1 day before and 6 days after transplant attained donor chimerism levels ranging from 15-35%. Further, a 14 day course of sirolimus was sufficient to maintain stable mixed chimerism in our model (See Figures 1 and 2). To examine whether this synergistic effect is mediated by regulatory T cells, we administered anti-CD25 monoclonal antibody (CD25 mAb), an agent known to transiently deplete these cells in vivo. Fifteen mice received 200cGy TBI, sirolimus, PT-Cy, and either no CD25 mab, CD25 mab (1mg IP) on 7 and 3 days before and 1 day after transplant, or CD25 mab starting 14 days after transplant. CD25 mab was given biweekly for 5 weeks to mice in both groups. Donor engraftment levels did not differ in the three groups, with donor chimerism levels ranging from 30-40%. Our data show that the anti-proliferative effects of sirolimus do not inhibit the efficacy of the cytotoxic agent cyclophosphamide. Rather, our data demonstrate that the combination of PT-cy and a limited course of sirolimus synergistically promote mixed bone marrow chimerism in a complete mismatched setting. Further, the synergistic effect of this drug combination appears to be mediated independently from CD25+ regulatory T cell expression. In light of our previous success using sirolimus in an HLA-matched HSCT protocol, these findings lay the groundwork for developing PT-cy and sirolimus as a novel, safe, and effective means of promoting stable mixed chimerism in the haploidentical setting and thus greatly enhancing our ability to successfully apply this approach to patients with severe SCD.
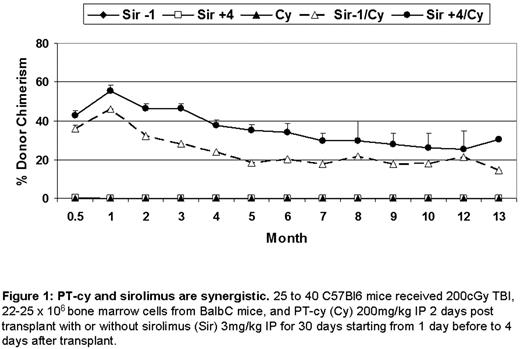
PT-cy and sirolimus are synergistic. 25 to 40 C57BI6 mice received 200cGy TBI, 22-25 × 106 bone marrow cells from BalbC mice, and PT-cy (Cy) 200mg/kg IP 2 days post transplant with or without sirolimus (Sir) 3mg/kg IP for 30 days starting from 1 day before to 4 days after transplant.
PT-cy and sirolimus are synergistic. 25 to 40 C57BI6 mice received 200cGy TBI, 22-25 × 106 bone marrow cells from BalbC mice, and PT-cy (Cy) 200mg/kg IP 2 days post transplant with or without sirolimus (Sir) 3mg/kg IP for 30 days starting from 1 day before to 4 days after transplant.
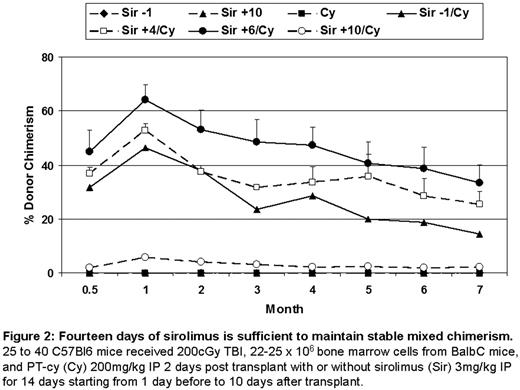
Fourteen days of sirolimus is sufficient to maintain stable mixed chimerism. 25 to 40 C57BI6 mice received 200cGy TBI, 22-25 × 106 bone marrow cells from BalbC mice, and PT-cy (Cy) 200mg/kg IP 2 days post transplant with or without sirolimus (Sir) 3mg/kg IP for 14 days starting from 1 day before to 10 days after transplant.
Fourteen days of sirolimus is sufficient to maintain stable mixed chimerism. 25 to 40 C57BI6 mice received 200cGy TBI, 22-25 × 106 bone marrow cells from BalbC mice, and PT-cy (Cy) 200mg/kg IP 2 days post transplant with or without sirolimus (Sir) 3mg/kg IP for 14 days starting from 1 day before to 10 days after transplant.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal