Abstract
Abstract 3357
Poster Board III-245
Reduced intensity (RIC) allo-HCT has been shown to reduce transplant-related mortality (TRM) but is associated with a higher risk of relapse when compared to myeloablative regimens in patients with aggressive NHL. Zevalin® offers the advantage of targeted radiation delivery to disease sites with less radiation exposure to normal organs. Zevalin® is an effective therapy for both relapsed low-grade and diffuse large B-cell lymphoma (DLBCL). Several studies have shown that Zevalin® can be added to a high-dose regimen in the autologous HCT setting without additional toxicity. We conducted a phase II study to determine whether adding Zevalin® to a RIC regimen of fludarabine and melphalan followed by allo-HCT can improve disease control thus reducing the risk of relapse in patients with relapsed and refractory B-cell NHL.
Patients received 111In- Zevalin® on day -21 followed by 90Y- Zevalin® 0.4mCi/kg delivered as an outpatient on day -14, fludarabine 25 mg/m2 daily x 5 days on days -9 to -5 and melphalan 140 mg/m2 on day -4. GVHD prophylaxis consisted of tacrolimus and sirolimus beginning on day-3. Short methotrexate was added for mismatched unrelated donor.
Between 10/2007 and 6/2009, 13 patients were enrolled. Eight were male and 5 female with the median age of 55 years (range, 27-67). The histologies included DLBCL= 5, transformed Lymphoma = 3, MCL blastoid variant=2, FL=2 and MZL=1. All patients except 2 with MCL had advanced refractory disease with the median number of 4 prior chemo regimens (range 2-7). One had prior auto-HCT. All patients received rituximab prior to allo-HCT. The median time from diagnosis to allo-HCT was 11 months (range, 3-90). Disease status at transplant; induction failure=6, relapse=4, CR=2, PR=1. Eleven were FDG-PET positive at the time of transplant. Donors were matched siblings in 5 and unrelated donors in 8. All patients received peripheral blood stem cells. The median dose of Zevalin® given was 32 mCi (range, 23-35).
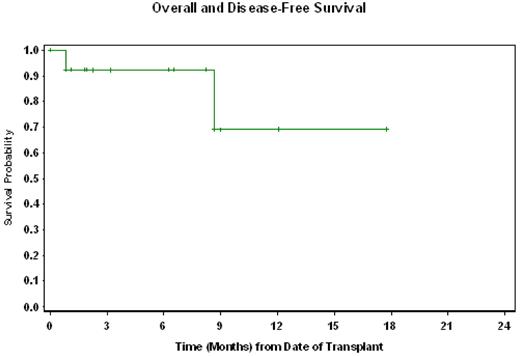
All patients engrafted at the median of 14 days (range 10-17) for ANC>500/μL and 19 days (range 16-20) for platelet > 20,000/μL. Engraftment analysis showed complete donor chimerism in all except one early death. The transplant-related toxicities were similar to fludarabine/melphalan regimen. There were 2 deaths in total. One patient died from viral pneumonia and diffuse alveolar hemorrhage at day +26 post-HCT. Another patient died at 9 months from sepsis. TRM was 8% and 31% at day 100 and 1-year respectively. Six (46%) developed grade II-IV acute GVHD and chronic GVHD. Of the11 with active disease (FDG-PET positive), 10 were evaluable for response. There were 6 CRs, 3 PRs and one stable disease at 30 days post-transplant. Thus far, none of the patients have relapsed. With a median follow-up for the surviving patients of 6 months (range 1-18), 11 (85%) are alive in remission. The estimated overall and disease-free survival probabilities at one-year are both 69% (95% CI, 33%-89%) Figure 1.
This study demonstrates the feasibility, tolerability and efficacy of adding Zevalin® to RIC fludarabine and melphalan in the allo-HCT setting for B-cell NHL. The response observed at 1 month post-transplant in patients with refractory disease are encouraging and suggest that this approach could be used to provide early disease control before graft-versus lymphoma effect takes place.
Nademanee:Genzyme Corporation: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal