Abstract
Abstract 3353
Poster Board III-241
Renal dysfunction is a life-threatening complication after hematopoietic stem cell transplantation, and the incidence of acute and chronic renal dysfunction after non-myeloablative transplantation is reportedly, 40 to 50% and 16 to 35%, respectively. In this study, we evaluated this complication after reduced-intensity stem cell transplantation (RIST) at a single institute.
We retrospectively reviewed the medical records of 286 patients (median age, 54 years; range, 21-68) with various hematological disorders who underwent RIST between 1999 and 2007, using a conditioning regimen that consisted of busulfan (oral 8 mg/kg or iv 6.4 mg/kg) and fludarabine (180 mg/m2, n=214) or cladribine (0.66 mg/kg, n=72). Sixty-seven patients also received 2-4 Gy of total body irradiation (TBI). The diagnosis included AML (n=77), ALL (n=9), MDS (n=56), malignant lymphoma (n=116), CML (n=14), and other (n=14). Whereas 188 patients were transplanted from a related donor (BM n=8, PB n=180), 98 were transplanted from an unrelated donor (BM n=80, PB n=1, CB n=17). GVHD prophylaxis consisted of cyclosporine (CSP, starting dose 3 mg/kg/day civ, target whole blood conc. 250-350 ng/ml, n=235) or tacrolimus (starting dose 0.03mg/kg/day civ, target whole blood conc. 10-20 ng/ml, n=51), with (n=131) or without (n=155) methotrexate, and 71 patients also received anti-human T-lymphocyte immunoglobulin (ATG, Fresenius, 5-10 mg/kg). Renal function was assessed in terms of the serum creatinine concentration and the estimated glomerular filtration rate (eGFR) as calculated by the Modification of Diet in Renal Disease equation (MDRD) for our population (eGFR=0.741*175*Age-0.203*Cr-1.154, *0.742, if female). Acute renal failure (ARF) within 100 days was categorized as grade 0 (decrease in eGFR of <25% of the baseline value), grade 1 (less than two-fold rise in the serum creatinine concentration with a decrease in eGFR of >25% but <50% of the baseline value), grade 2 (greater than two-fold rise in the serum creatinine concentration compared to the baseline value but not requiring dialysis), and grade 3 (same as grade 2 but requiring dialysis). Chronic kidney disease (CKD) was defined as two or more estimates of eGFR <60 ml/min/1.73 m2 within a minimum 30-day interval after day 100. Patients with eGFR <60 at baseline and/or less than 131 days of follow-up (n=121) were excluded from the CKD study cohort, which left 165 patients eligible for analysis.
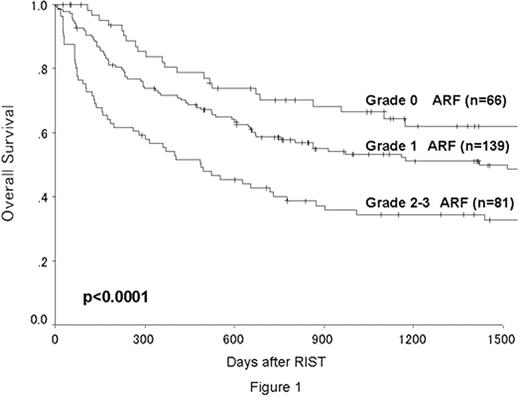
The median follow-up in surviving patients was 746 days (87-3291). The numbers of patients who developed grade 1, 2 and 3 ARF were 139, 72 and 9, respectively. The cumulative incidence of grade 2-3 ARF was 29 % at 100 days after RIST. The overall survival was significantly better in patients with grade 0-1 ARF than in those with grade 2-3 ARF (62% vs 42% at 2 years, p<0.0001, Figure 1). There was no significant difference in the occurrence of ARF between the cladribine and fludarabine groups (p=0.28). Multivariate analyses for ARF showed that TBI and CSP were significantly associated with a higher incidence of grade 2-3 ARF. CKD developed in 15 of the 165 evaluable patients (9.1%), and all had received PBSCT and CSP for GVHD prophylaxis. The cumulative incidence of CKD was 3.8% at 1 year, 9.0% at 2 years and 11.7% at 3 years after RIST. Grade 2-3 ARF (p=0.014) and the development of chronic GVHD (p=0.048) were significantly associated with a higher incidence of CKD. There was no significant difference in survival between patients with and without CKD (p=0.16).
We found that ARF was significantly associated with a higher mortality after busulfan-based RIST, and that preceding grade 2-3 ARF led to the occurrence of CKD in surviving patients, which highlights the importance of preventing ARF. This study also indicated that the incidence of acute and chronic renal complications was lower in our population compared to previously reported data from Western countries. Therefore, ethnicity should be taken into account when designing future international clinical trials.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal