Abstract
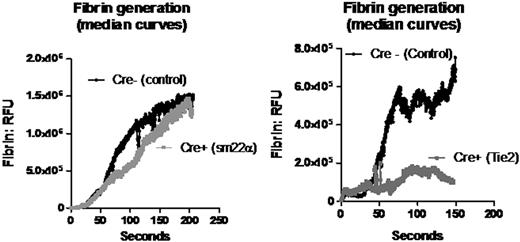
Tissue factor (TF) is required for the initiation of thrombin formation and fibrin generation in vivo. However, the functional role of intravascular tissue factor in thrombus formation is the subject of ongoing controversy. To address this question, we compared fibrin generation and platelet thrombus formation between mice lacking tissue factor in either the intravascular or the extravascular compartment using the laser injury model of thrombosis in the mouse cremaster muscle. Laser injury initiation was chosen for three reasons: (i) thrombus initiation in this model has been shown to follow endothelial activation as measured by an increase in intracellular calcium in the endothelium following laser injury; (ii) thrombus formation in this model is dominated by activation of platelets by thrombin rather than collagen; (iii) endothelial disruption by laser injury histologically appears minimal compared with thrombus initiation by ferric chloride, Rose Bengal or mechanical disruption. In this study, TF was selectively deleted in tissues of intravascular versus extravascular origin using the Cre-lox method of targeted gene deletion. TFflox/flox mice were crossed with Tie2Cre+/o mice for combined endothelial and hematopoietic TF deletion, and sm22αCre+/o mice for smooth muscle cell TF deletion. In the Tie2Cre+ TFflox/flox mice, TF DNA was reduced by 93% in the whole blood compared with Cre- controls. In the aortic media of sm22αCre+ TFflox/flox mice, TF mRNA was 96% decreased and TF activity was reduced by 95% compared with control, while there was no difference in circulating TF activity (Wang et al, Blood. 2009;113). Using the laser injury model of thrombosis in the cremaster arteriole of a live mouse, thrombus and fibrin generation were evaluated using intravital multichannel fluorescence and brightfield widefield microscopy. Platelet accumulation was measured using either an Alexa-649 labeled anti-mouse GP1bβ antibody, or Fab fragments of an Alexa-647 labeled anti-mouse GPIIbIIIa antibody directed against CD41. Fibrin formation was measured using an Alexa-488 labeled fibrin-specific antibody. TF deletion in endothelial and hematopoietic tissue resulted in a marked decrease in maximal fibrin generation (82%, p=0.004) compared with Cre- control mice. Maximal thrombus size was decreased by 55% (p=0.04). In contrast, TF deletion in the smooth muscle cell compartment resulted in no change in fibrin formation compared with Cre- control. We conclude that when endothelial disruption is minimized, such as in the laser injury model of thrombosis, intravascular TF plays a significant role in fibrin formation, while smooth muscle cell derived TF does not. Although these models of thrombus formation have no direct parallel in human disease, it is likely that the pathogenesis of thrombosis in humans include some mechanisms in which extravascular tissue factor plays a dominant role and others in which intravascular tissue factor plays a dominant role.
No relevant conflicts of interest to declare.

This icon denotes an abstract that is clinically relevant.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal